Exosomes function in antigen presentation during an in vivo Mycobacterium tuberculosis infection
- PMID: 28262829
- PMCID: PMC5338015
- DOI: 10.1038/srep43578
Exosomes function in antigen presentation during an in vivo Mycobacterium tuberculosis infection
Abstract
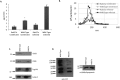
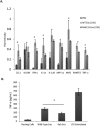
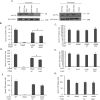
Mycobacterium tuberculosis-infected macrophages and dendritic cells are limited in their ability to present antigen to CD4+ T cells suggesting that other mechanism of antigen presentation are driving the robust T cell response observed during an M. tuberculosis infection. These mechanisms could include antigens present in apoptotic bodies, necrotic debris, exosomes or even release of non-vesicular antigen from infected cells. However, there is limited data to support any of these mechanisms as important in driving T cell activation in vivo. In the present study we use Rab27a-deficient mice which show diminished trafficking of mycobacterial components to exosomes as well as M. tuberculosis strains that express recombinant proteins which traffic or fail to traffic to exosomes. We observed that exosomes released during a mouse M. tuberculosis infection contribute significantly to its T cell response. These finding imply that exosomes function to promote T cell immunity during a bacterial infection and are an important source of extracellular antigen.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Suboptimal Antigen Presentation Contributes to Virulence of Mycobacterium tuberculosis In Vivo.J Immunol. 2016 Jan 1;196(1):357-64. doi: 10.4049/jimmunol.1501494. Epub 2015 Nov 16. J Immunol. 2016. PMID: 26573837 Free PMC article.
-
Exosomes isolated from mycobacteria-infected mice or cultured macrophages can recruit and activate immune cells in vitro and in vivo.J Immunol. 2012 Jul 15;189(2):777-85. doi: 10.4049/jimmunol.1103638. Epub 2012 Jun 20. J Immunol. 2012. PMID: 22723519 Free PMC article.
-
Microparticles from mycobacteria-infected macrophages promote inflammation and cellular migration.J Immunol. 2013 Jan 15;190(2):669-77. doi: 10.4049/jimmunol.1201856. Epub 2012 Dec 14. J Immunol. 2013. PMID: 23241892
-
CD1d and natural killer T cells in immunity to Mycobacterium tuberculosis.Adv Exp Med Biol. 2013;783:199-223. doi: 10.1007/978-1-4614-6111-1_11. Adv Exp Med Biol. 2013. PMID: 23468111 Review.
-
Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis.Immunol Rev. 2015 Mar;264(1):220-32. doi: 10.1111/imr.12268. Immunol Rev. 2015. PMID: 25703562 Review.
Cited by
-
Non-Exosomal and Exosome-Derived miRNAs as Promising Biomarkers in Canine Mammary Cancer.Life (Basel). 2022 Apr 1;12(4):524. doi: 10.3390/life12040524. Life (Basel). 2022. PMID: 35455015 Free PMC article. Review.
-
Immune cells-derived exosomes function as a double-edged sword: role in disease progression and their therapeutic applications.Biomark Res. 2022 May 12;10(1):30. doi: 10.1186/s40364-022-00374-4. Biomark Res. 2022. PMID: 35550636 Free PMC article. Review.
-
Current landscape of exosomes in tuberculosis development, diagnosis, and treatment applications.Front Immunol. 2024 May 23;15:1401867. doi: 10.3389/fimmu.2024.1401867. eCollection 2024. Front Immunol. 2024. PMID: 38846947 Free PMC article. Review.
-
Host Long Noncoding RNAs as Key Players in Mycobacteria-Host Interactions.Microorganisms. 2024 Dec 21;12(12):2656. doi: 10.3390/microorganisms12122656. Microorganisms. 2024. PMID: 39770858 Free PMC article. Review.
-
Bedaquiline reprograms central metabolism to reveal glycolytic vulnerability in Mycobacterium tuberculosis.Nat Commun. 2020 Nov 30;11(1):6092. doi: 10.1038/s41467-020-19959-4. Nat Commun. 2020. PMID: 33257709 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

