Prophylactic levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease
- PMID: 28262914
- PMCID: PMC6464336
- DOI: 10.1002/14651858.CD011312.pub2
Prophylactic levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease
Update in
-
Prophylactic levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease.Cochrane Database Syst Rev. 2017 Aug 2;8(8):CD011312. doi: 10.1002/14651858.CD011312.pub3. Cochrane Database Syst Rev. 2017. PMID: 28770972 Free PMC article.
Abstract
Background: Low cardiac output syndrome remains a serious complication, and accounts for substantial morbidity and mortality in the postoperative course of paediatric patients undergoing surgery for congenital heart disease. Standard prophylactic and therapeutic strategies for low cardiac output syndrome are based mainly on catecholamines, which are effective drugs, but have considerable side effects. Levosimendan, a calcium sensitiser, enhances the myocardial function by generating more energy-efficient myocardial contractility than achieved via adrenergic stimulation with catecholamines. Thus potentially, levosimendan is a beneficial alternative to standard medication for the prevention of low cardiac output syndrome in paediatric patients after open heart surgery.
Objectives: To review the efficacy and safety of the postoperative prophylactic use of levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease.
Search methods: We identified trials via systematic searches of CENTRAL, MEDLINE, Embase, and Web of Science, as well as clinical trial registries, in June 2016. Reference lists from primary studies and review articles were checked for additional references.
Selection criteria: We only included randomised controlled trials (RCT) in our analysis that compared prophylactic levosimendan with standard medication or placebo, in infants and children up to 18 years of age, who were undergoing surgery for congenital heart disease.
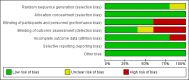
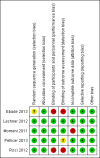
Data collection and analysis: Two review authors independently extracted data and assessed risk of bias according to a pre-defined protocol. We obtained additional information from all but one of the study authors of the included studies. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of evidence from the studies that contributed data to the meta-analyses for the prespecified outcomes. We created a 'Summary of findings' table to summarise the results and the quality of evidence for each outcome.
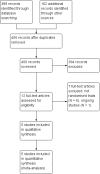
Main results: We included five randomised controlled trials with a total of 212 participants in the analyses. All included participants were under five years of age. Using GRADE, we assessed there was low-quality evidence for all analysed outcomes. We assessed high risk of performance and detection bias for two studies due to their unblinded setting. Levosimendan showed no clear effect on risk of mortality (risk ratio (RR) 0.47, 95% confidence interval (CI) 0.12 to 1.82; participants = 123; studies = 3) and no clear effect on low cardiac output syndrome (RR 0.64, 95% CI 0.39 to 1.04; participants = 83; studies = 2) compared to standard treatments. Data on time-to-death were not available from any of the included studies.There was no conclusive evidence on the effect of levosimendan on the secondary outcomes. The levosimendan groups had shorter length of intensive care unit stays (mean difference (MD) 0.33 days, 95% CI -1.16 to 1.82; participants = 188; studies = 4; I² = 35%), length of hospital stays (0.26 days, 95% CI -3.50 to 4.03; participants = 75; studies = 2), and duration of mechanical ventilation (MD -0.04 days, 95% CI -0.08 to 0.00; participants = 208; studies = 5; I² = 0%). The risk of mechanical circulatory support or cardiac transplantation favoured the levosimendan groups (RR 1.49, 95% CI 0.19 to 11.37; participants = 60; studies = 2). Published data about adverse effects of levosimendan were limited. A meta-analysis of hypotension, one of the most feared side effects of levosimendan, was not feasible because of the heterogeneous expression of blood pressure values.
Authors' conclusions: The current level of evidence is insufficient to judge whether prophylactic levosimendan prevents low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease. So far, no significant differences have been detected between levosimendan and standard inotrope treatments in this setting.The authors evaluated the quality of evidence as low, using the GRADE approach. Reasons for downgrading were serious risk of bias (performance and detection bias due to unblinded setting of two RCTs), serious risk of inconsistency, and serious to very serious risk of imprecision (small number of included patients, low event rates).
Conflict of interest statement
JH: None known.
GR: received payment for a one‐day course on statistical methods in meta‐analysis by Grünenthal Group, Aachen, Germany.
BS: None known.
Figures
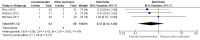









Similar articles
-
Prophylactic levosimendan for the prevention of low cardiac output syndrome and mortality in paediatric patients undergoing surgery for congenital heart disease.Cochrane Database Syst Rev. 2017 Aug 2;8(8):CD011312. doi: 10.1002/14651858.CD011312.pub3. Cochrane Database Syst Rev. 2017. PMID: 28770972 Free PMC article.
-
Prophylactic use of inotropic agents for the prevention of low cardiac output syndrome and mortality in adults undergoing cardiac surgery.Cochrane Database Syst Rev. 2024 Nov 27;11(11):CD013781. doi: 10.1002/14651858.CD013781.pub2. Cochrane Database Syst Rev. 2024. PMID: 39601298 Free PMC article.
-
Inotropes for the prevention of low cardiac output syndrome and mortality for paediatric patients undergoing surgery for congenital heart disease: a network meta-analysis.Cochrane Database Syst Rev. 2024 Nov 26;11(11):CD013707. doi: 10.1002/14651858.CD013707.pub2. Cochrane Database Syst Rev. 2024. PMID: 39588800 Free PMC article. Review.
-
Prophylactic milrinone for the prevention of low cardiac output syndrome and mortality in children undergoing surgery for congenital heart disease.Cochrane Database Syst Rev. 2015 Mar 25;2015(3):CD009515. doi: 10.1002/14651858.CD009515.pub2. Cochrane Database Syst Rev. 2015. PMID: 25806562 Free PMC article.
-
Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome.Cochrane Database Syst Rev. 2018 Jan 29;1(1):CD009669. doi: 10.1002/14651858.CD009669.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2020 Nov 5;11:CD009669. doi: 10.1002/14651858.CD009669.pub4. PMID: 29376560 Free PMC article. Updated.
Cited by
-
Congenital Heart Disease: The State-of-the-Art on Its Pharmacological Therapeutics.J Cardiovasc Dev Dis. 2022 Jun 26;9(7):201. doi: 10.3390/jcdd9070201. J Cardiovasc Dev Dis. 2022. PMID: 35877563 Free PMC article. Review.
-
An Update on Pharmacologic Management of Neonatal Hypotension: When, Why, and Which Medication.Children (Basel). 2024 Apr 19;11(4):490. doi: 10.3390/children11040490. Children (Basel). 2024. PMID: 38671707 Free PMC article. Review.
-
Effect of Preoperative Infusion of Levosimendan on Biomarkers of Myocardial Injury and Haemodynamics After Paediatric Cardiac Surgery: A Randomised Controlled Trial.Drugs R D. 2021 Mar;21(1):79-89. doi: 10.1007/s40268-020-00332-1. Epub 2020 Dec 24. Drugs R D. 2021. PMID: 33367965 Free PMC article. Clinical Trial.
-
The Perspective of the Intensivist on Inotropes and Postoperative Care Following Pediatric Heart Surgery: An International Survey and Systematic Review of the Literature.World J Pediatr Congenit Heart Surg. 2018 Jan;9(1):10-21. doi: 10.1177/2150135117731725. Epub 2017 Nov 1. World J Pediatr Congenit Heart Surg. 2018. PMID: 29092664 Free PMC article.
-
Clinical efficacy of levosimendan vs milrinone in preventing low cardiac output syndrome following pediatric cardiac surgery.Ann Card Anaesth. 2021 Apr-Jun;24(2):217-223. doi: 10.4103/aca.ACA_160_19. Ann Card Anaesth. 2021. PMID: 33884979 Free PMC article. Clinical Trial.
References
References to studies included in this review
References to studies excluded from this review
-
- Giordano R, Palma G, Palumbo S, Cioffi S, Russolillo V, Mucerino M, et al. Single center experience with levosimendan administration after pediatric cardiac surgery. Giornale Italiano di Cardiologia / Abstract del XLIII Congresso Nazionale SICP ‐ Sezione Pediatrica e delle Cardiopatie Congenite SICCH 2013;14(Suppl 1 AL N 10):35S‐6S.
References to ongoing studies
-
- EUCTR 2012‐005310‐19‐ES. Double‐blind randomized clinical trial to evaluate the efficacy and safety of levosimendan as preischemic myocardial conditioner in pediatric cardiac surgery. www.clinicaltrialsregister.eu/ctr‐search/search?query=EUCTR2012‐005310‐1... (accessed 26 November 2014).
Additional references
-
- Bailey J, Hoffman T, Wessel D, Nelson D, Atz A, Chang A, et al. A population pharmacokinetic analysis of milrinone in pediatric patients after cardiac surgery. Journal of Pharmacokinetics and Pharmacodynamics 2004;31(1):43‐59. - PubMed
-
- Baysal A, Sasmazel A, Yildirim A, Kocak Tu, Sunar H, Zeybek R. The effects of thyroid hormones and interleukin‐8 levels on prognosis after congenital heart surgery. Archives of the Turkish Society of Cardiology 2010;38(8):537‐43. - PubMed
-
- Braun J‐P, Schneider M, Kastrup M, Liu J. Treatment of acute heart failure in an infant after cardiac surgery using levosimendan. European Journal of Cardio‐thoracic Surgery 2004;26(1):228‐30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

