Iron-deficient erythropoiesis in blood donors and red blood cell recovery after transfusion: initial studies with a mouse model
- PMID: 28263174
- PMCID: PMC5336338
- DOI: 10.2450/2017.0349-16
Iron-deficient erythropoiesis in blood donors and red blood cell recovery after transfusion: initial studies with a mouse model
Abstract
Background: Most frequent red cell (RBC) donors and many first-time donors are iron deficient, but meet haemoglobin standards. However, the effects of donation-induced iron deficiency on RBC storage quality are unknown. Thus, we used a mouse model to determine if donor iron deficiency reduced post-transfusion RBC recovery.
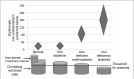
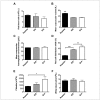
Methods: Weanling mice received a control diet or an iron-deficient diet. A third group receiving the iron-deficient diet was also phlebotomised weekly. This provided 3 groups of mice with different iron status: (1) iron replete, (2) mild iron deficiency with iron-deficient erythropoiesis, and (3) iron-deficiency anaemia. At ten weeks of age, blood was collected, leucoreduced, and stored at 4 ºC. After 12 days of storage, 24-hour (h) post-transfusion RBC recovery was quantified in recipients by flow cytometry.
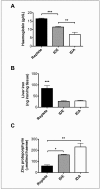
Results: Before blood collection, mean haemoglobin concentrations in the iron-replete, iron-deficient, and iron-deficiency anaemia donor mice were 16.5±0.4, 11.5±0.4, and 7.0±1.4 [g/dL± 1 standard deviation (SD)], respectively (p<0.01 for all comparisons between groups). The 24-h post-transfusion RBC recoveries in recipients receiving transfusions from these three cohorts were 77.1±13.2, 66.5±10.9, and 46.7±15.9 (% ±1 SD), respectively (p<0.05 for all comparisons between groups).
Discussion: In summary, donor iron deficiency significantly reduced 24-h post-transfusion RBC recovery in recipient mice. RBCs from mice with mild iron deficiency and iron-deficient erythropoiesis, with haemoglobin levels similar to those used for human autologous blood donation, had intermediate post-transfusion RBC recovery, as compared to iron-replete donors and those with iron-deficiency anaemia. This suggests that, in addition to the effects of iron deficiency on donor health, frequent blood donation, leading to iron-deficient erythropoiesis, may also have adverse effects for transfusion recipients.
Conflict of interest statement
The Authors declare no conflicts of interest.
Figures

References
-
- Brittenham GM. Disorders of iron homeostasis: iron deficiency and overload. In: Hoffman R, Benz EJ, Silberstein LE, et al., editors. Hematology. 6th ed. Philadelphia: Elsevier/Saunders; 2013. pp. 437–49.
-
- Hastka J, Lasserre J-J, Schwarzbeck A, et al. Laboratory tests of iron status: correlation or common sense? Clin Chem. 1996;42:718–24. - PubMed
-
- Smith GA, Fisher SA, Doree C, Roberts DJ. A systematic review of factors associated with the deferral of donors failing to meet low haemoglobin thresholds. Transfusion Med. 2013;23:309–20. - PubMed
-
- Birgegard G, Schneider K, Ulfberg J. High incidence of iron depletion and restless leg syndrome (RLS) in regular blood donors: intravenous iron sucrose substitution more effective than oral iron. Vox Sang. 2010;99:354–61. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
