The purified vepoloxamer prevents haemolysis in 42-day stored, DEHP/PVC-free red blood cell units
- PMID: 28263175
- PMCID: PMC5336339
- DOI: 10.2450/2017.0351-16
The purified vepoloxamer prevents haemolysis in 42-day stored, DEHP/PVC-free red blood cell units
Abstract
Background: Use of the plasticiser di(2-ethylhexyl) phthalate (DEHP) in polyvinyl chloride (PVC) blood bags poses a potential dilemma. The presence of DEHP in blood bags has been shown to be beneficial to red blood cells during storage by diminishing haemolysis. However, DEHP use in PVC may be carcinogenic or estrogenising. Vepoloxamer is a poloxamer with rheological and cytoprotective rheological properties and a favourable toxicity profile in clinical trials. We hypothesised that vepoloxamer may be sufficient to replace the plasticiser DEHP to prevent elevated haemolysis while conserving the biochemical and redox potential++ in RBCs stored for up to 42 days.
Materials and methods: Paired analyses of aliquots from pooled RBC suspensions of ABO identical donors were aseptically split into test storage containers (DEHP/PVC or DEHP-free/ethylene vinyl acetate [EVA]) supplemented with or without vepoloxamer (at concentrations of 0.1, 1, 5 or 7.89 mg/mL) and cold stored for up to 42 days.
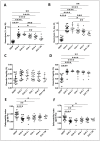
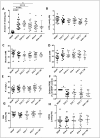
Results: Vepoloxamer significantly prevented the increased haemolysis induced by the absence of DEHP in EVA bags in a dose-dependent manner by days 28 and 42 of storage (approx. 50% reduction of the maximum concentration of vepoloxamer; p<0.001). There was an inverse correlation between the concentration of vepoloxamer used and the haemolysis rate (r2=0.27, p<0.001) and a direct correlation between haemolysis and phosphatidylserine (PS) exposure (r2=0.42; p<0.01). Increased osmotic fragility and shear induced deformability of 42-day stored RBC in EVA bags was significantly corrected by the addition of vepoloxamer.
Discussion: Vepoloxamer, in a concentration-dependent fashion, is able to partly rescue the increased haemolysis and PS exposure induced by the absence of the commonly used plasticiser DEHP. These results provide initial but strong evidence to support vepoloxamer use to replace DEHP in long-term storage of RBC.
Conflict of interest statement
RME and DSMc-K are employees of Mast Therapeutics Inc. Mast Therapeutics Inc. provided study materials and funds to conduct this study. The other Authors declare no conflicts of interest.
Figures
References
-
- Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210:623–34. - PubMed
-
- Scientific Committee on Emerging and Newly-Identified Health Risks. Preliminary report on the safety of medical devices containing DEHP-plasticized PVC or other plasticizers on neonates and other groups possibly at risk. Health & Consumer Protection, European Commission; 2007. [Accessed on 17/01/2017]. Available at: http://ec.europa.eu/health/ph_risk/committees/04_scenihr/docs/scenihr_o_....
-
- Food and Drug Administration, Health CfDaR. Safety assessment of di(2-ethylhexyl)phthalate (DEHP) released from PVC medical devices. 2002. [Accessed on 17/01/2017]. Available at: DeviceRegulationandGuidance/GuidanceDocuments/UCM080457.pdf.
-
- Jaeger RJ, Rubin RJ. Plasticizers from plastic devices extraction, metabolism, and accumulation by biological systems. Science. 1970;170:460–2. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
