Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism
- PMID: 28263184
- PMCID: PMC5373887
- DOI: 10.1172/JCI88641
Angiotensin AT1A receptors on leptin receptor-expressing cells control resting metabolism
Abstract
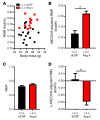
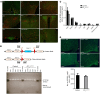
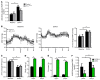
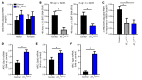
Leptin contributes to the control of resting metabolic rate (RMR) and blood pressure (BP) through its actions in the arcuate nucleus (ARC). The renin-angiotensin system (RAS) and angiotensin AT1 receptors within the brain are also involved in the control of RMR and BP, but whether this regulation overlaps with leptin's actions is unclear. Here, we have demonstrated the selective requirement of the AT1A receptor in leptin-mediated control of RMR. We observed that AT1A receptors colocalized with leptin receptors (LEPRs) in the ARC. Cellular coexpression of AT1A and LEPR was almost exclusive to the ARC and occurred primarily within neurons expressing agouti-related peptide (AgRP). Mice lacking the AT1A receptor specifically in LEPR-expressing cells failed to show an increase in RMR in response to a high-fat diet and deoxycorticosterone acetate-salt (DOCA-salt) treatments, but BP control remained intact. Accordingly, loss of RMR control was recapitulated in mice lacking AT1A in AgRP-expressing cells. We conclude that angiotensin activates divergent mechanisms to control BP and RMR and that the brain RAS functions as a major integrator for RMR control through its actions at leptin-sensitive AgRP cells of the ARC.
Conflict of interest statement
Figures



Comment in
-
Worth its salt.Sci Transl Med. 2017 Apr 5;9(384):eaan2774. doi: 10.1126/scitranslmed.aan2774. Sci Transl Med. 2017. PMID: 28381536
References
-
- Dinh DT, Frauman AG, Johnston CI, Fabiani ME. Angiotensin receptors: distribution, signalling and function. Clin Sci. 2001;100(5):481–492. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

