Laminar flow downregulates Notch activity to promote lymphatic sprouting
- PMID: 28263185
- PMCID: PMC5373895
- DOI: 10.1172/JCI87442
Laminar flow downregulates Notch activity to promote lymphatic sprouting
Abstract
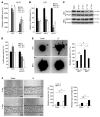
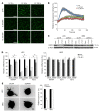
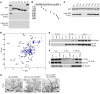
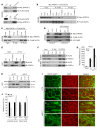
The major function of the lymphatic system is to drain interstitial fluid from tissue. Functional drainage causes increased fluid flow that triggers lymphatic expansion, which is conceptually similar to hypoxia-triggered angiogenesis. Here, we have identified a mechanotransduction pathway that translates laminar flow-induced shear stress to activation of lymphatic sprouting. While low-rate laminar flow commonly induces the classic shear stress responses in blood endothelial cells and lymphatic endothelial cells (LECs), only LECs display reduced Notch activity and increased sprouting capacity. In response to flow, the plasma membrane calcium channel ORAI1 mediates calcium influx in LECs and activates calmodulin to facilitate a physical interaction between Krüppel-like factor 2 (KLF2), the major regulator of shear responses, and PROX1, the master regulator of lymphatic development. The PROX1/KLF2 complex upregulates the expression of DTX1 and DTX3L. DTX1 and DTX3L, functioning as a heterodimeric Notch E3 ligase, concertedly downregulate NOTCH1 activity and enhance lymphatic sprouting. Notably, overexpression of the calcium reporter GCaMP3 unexpectedly inhibited lymphatic sprouting, presumably by disturbing calcium signaling. Endothelial-specific knockouts of Orai1 and Klf2 also markedly impaired lymphatic sprouting. Moreover, Dtx3l loss of function led to defective lymphatic sprouting, while Dtx3l gain of function rescued impaired sprouting in Orai1 KO embryos. Together, the data reveal a molecular mechanism underlying laminar flow-induced lymphatic sprouting.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

