CD11b activation suppresses TLR-dependent inflammation and autoimmunity in systemic lupus erythematosus
- PMID: 28263189
- PMCID: PMC5373862
- DOI: 10.1172/JCI88442
CD11b activation suppresses TLR-dependent inflammation and autoimmunity in systemic lupus erythematosus
Abstract
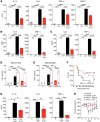
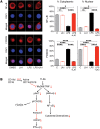
Genetic variations in the ITGAM gene (encoding CD11b) strongly associate with risk for systemic lupus erythematosus (SLE). Here we have shown that 3 nonsynonymous ITGAM variants that produce defective CD11b associate with elevated levels of type I interferon (IFN-I) in lupus, suggesting a direct link between reduced CD11b activity and the chronically increased inflammatory status in patients. Treatment with the small-molecule CD11b agonist LA1 led to partial integrin activation, reduced IFN-I responses in WT but not CD11b-deficient mice, and protected lupus-prone MRL/Lpr mice from end-organ injury. CD11b activation reduced TLR-dependent proinflammatory signaling in leukocytes and suppressed IFN-I signaling via an AKT/FOXO3/IFN regulatory factor 3/7 pathway. TLR-stimulated macrophages from CD11B SNP carriers showed increased basal expression of IFN regulatory factor 7 (IRF7) and IFN-β, as well as increased nuclear exclusion of FOXO3, which was suppressed by LA1-dependent activation of CD11b. This suggests that pharmacologic activation of CD11b could be a potential mechanism for developing SLE therapeutics.
Conflict of interest statement
Figures





Comment in
-
Systemic lupus erythematosus: Defective CD11b raises IFN levels in SLE.Nat Rev Rheumatol. 2017 May;13(5):259. doi: 10.1038/nrrheum.2017.44. Epub 2017 Mar 23. Nat Rev Rheumatol. 2017. PMID: 28331209 No abstract available.
References
-
- International Consortium for Systemic Lupus Erythematosus Genetics (SLEGEN) et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40(2):204–210. doi: 10.1038/ng.81. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

