The Treatment of Possible Severe Infection in Infants: An Open Randomized Safety Trial of Parenteral Benzylpenicillin and Gentamicin Versus Ceftriaxone in Infants <60 days of Age in Malawi
- PMID: 28263245
- PMCID: PMC5466153
- DOI: 10.1097/INF.0000000000001576
The Treatment of Possible Severe Infection in Infants: An Open Randomized Safety Trial of Parenteral Benzylpenicillin and Gentamicin Versus Ceftriaxone in Infants <60 days of Age in Malawi
Abstract
Background: The World Health Organization recommends benzylpenicillin and gentamicin as antimicrobial treatment for infants with sepsis in low-income settings, and ceftriaxone or cefotaxime as an alternative. In a meta-analysis from 13 low-income settings, Staphylococcus aureus, Klebsiella spp. and Escherichia coli accounted for 55% of infants with sepsis. In a review of bacterial meningitis, resistance to third generation cephalosporins was >50% of all isolates, and 44% of Gram-negative isolates were gentamicin resistant. However, ceftriaxone may cause neonatal jaundice, and gentamicin may cause deafness. Therefore, we compared parenteral benzylpenicillin plus gentamicin with ceftriaxone as first-line treatment, assessing outcome and adverse events.
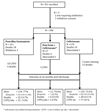
Methods: This was an open randomized trial carried out in the Queen Elizabeth Central Hospital, Blantyre, Malawi, from 2010 to 2013. Infants <60 days of age with possible severe sepsis received either benzylpenicillin and gentamicin or ceftriaxone. Adverse events and outcomes were recorded until 6 months post discharge.
Results: Three-hundred forty-eight infants were included in analyses. Outcome in the benzylpenicillin and gentamicin and ceftriaxone groups was similar; deaths were 13.7% and 16.5% and sequelae were 14.5% and 11.2%, respectively. More infants in the penicillin/gentamicin group required phototherapy: 15% versus 5%, P = 0.03. Thirteen (6%) survivors had bilateral hearing loss. There was no difference between the treatment groups. By 6 months post discharge, 11 more infants had died, and 17 more children were found to have sequelae.
Conclusions: Ceftriaxone and gentamicin are safe for infants in our setting. Infants should receive long-term follow-up as many poor outcomes occurred after hospital discharge.
Conflict of interest statement
The authors declare no conflicts of interest
References
-
- WHO Pocket Book of hospital care for children Guidelines for the management of common illnesses with limited resources. 2nd edition. World Health Organisation Geneva: 2013.
-
- The WHO young infants study group. Bacterial etiology of serious infections in young infants in developing countries: results of a multicenter study. PIDJ. 1999;18(supplement):S17–S22. - PubMed
-
- Downie L, Armiento R, Subhi R, et al. Community- acquired neonatal and infant sepsis in developing countries: efficacy of WHO's currently recommended antibiotics: systematic review and meta-analysis. Arch Dis Child. 2013;98(2):146–54. - PubMed
-
- Huynh B-T, Padget M, Garin B, Herindrainy P, Kermorvant-Duchemin E, Watier L, Guillemot D, Delarocque-Astagneau E. Burden of bacterial resistance among neonatal infections in low income countries: how convincing is the epidemiological evidence? BMC Inf Dis. 2015 doi: 10.1186/s12879-015-0843-x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous