Fixed-dose combination therapy for the prevention of atherosclerotic cardiovascular diseases
- PMID: 28263370
- PMCID: PMC6464321
- DOI: 10.1002/14651858.CD009868.pub3
Fixed-dose combination therapy for the prevention of atherosclerotic cardiovascular diseases
Abstract
Background: Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death and disability worldwide, yet ASCVD risk factor control and secondary prevention rates remain low. A fixed-dose combination of blood pressure- and cholesterol-lowering and antiplatelet treatments into a single pill, or polypill, has been proposed as one strategy to reduce the global burden of ASCVD.
Objectives: To determine the effect of fixed-dose combination therapy on all-cause mortality, fatal and non-fatal ASCVD events, and adverse events. We also sought to determine the effect of fixed-dose combination therapy on blood pressure, lipids, adherence, discontinuation rates, health-related quality of life, and costs.
Search methods: We updated our previous searches in September 2016 of CENTRAL, MEDLINE, Embase, ISI Web of Science, and DARE, HTA, and HEED. We also searched two clinical trials registers in September 2016. We used no language restrictions.
Selection criteria: We included randomised controlled trials of a fixed-dose combination therapy including at least one blood pressure-lowering and one lipid-lowering component versus usual care, placebo, or an active drug comparator for any treatment duration in adults 18 years old or older, with no restrictions on presence or absence of pre-existing ASCVD.
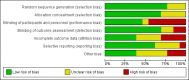
Data collection and analysis: Three review authors independently selected studies for inclusion and extracted the data for this update. We evaluated risk of bias using the Cochrane 'Risk of bias' assessment tool. We calculated risk ratios (RR) for dichotomous data and mean differences (MD) for continuous data with 95% confidence intervals (CI) using fixed-effect models when heterogeneity was low (I2 < 50%) and random-effects models when heterogeneity was high (I2 ≥ 50%). We used the GRADE approach to evaluate the quality of evidence.
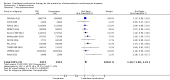
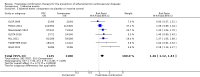
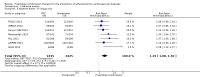
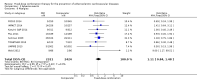
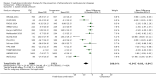
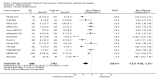
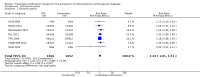
Main results: In the initial review, we identified nine randomised controlled trials with a total of 7047 participants and four additional trials (n = 2012 participants; mean age range 62 to 63 years; 30% to 37% women) were included in this update. Eight of the 13 trials evaluated the effects of fixed-dose combination (FDC) therapy in populations without prevalent ASCVD, and the median follow-up ranged from six weeks to 23 months. More recent trials were generally larger with longer follow-up and lower risk of bias. The main risk of bias was related to lack of blinding of participants and personnel, which was inherent to the intervention. Compared with the comparator groups (placebo, usual care, or active drug comparator), the effects of the fixed-dose combination treatment on mortality (FDC = 1.0% versus control = 1.0%, RR 1.10, 95% CI 0.64 to 1.89, I2 = 0%, 5 studies, N = 5300) and fatal and non-fatal ASCVD events (FDC = 4.7% versus control = 3.7%, RR 1.26, 95% CI 0.95 to 1.66, I2 = 0%, 6 studies, N = 4517) were uncertain (low-quality evidence). The low event rates for these outcomes and indirectness of evidence for comparing fixed-dose combination to usual care versus individual drugs suggest that these results should be viewed with caution. Adverse events were common in both the intervention (32%) and comparator (27%) groups, with participants randomised to fixed-dose combination therapy being 16% (RR 1.16, 95% CI 1.09 to 1.25, 11 studies, 6906 participants, moderate-quality evidence) more likely to report an adverse event . The mean differences in systolic blood pressure between the intervention and control arms was -6.34 mmHg (95% CI -9.03 to -3.64, 13 trials, 7638 participants, moderate-quality evidence). The mean differences (95% CI) in total and LDL cholesterol between the intervention and control arms were -0.61 mmol/L (95% CI -0.88 to -0.35, 11 trials, 6565 participants, low-quality evidence) and -0.70 mmol/L (95% CI -0.98 to -0.41, 12 trials, 7153 participants, moderate-quality evidence), respectively. There was a high degree of statistical heterogeneity in comparisons of blood pressure and lipids (I2 ≥ 80% for all) that could not be explained, so these results should be viewed with caution. Fixed-dose combination therapy improved adherence to a multidrug strategy by 44% (26% to 65%) compared with usual care (4 trials, 3835 participants, moderate-quality evidence).
Authors' conclusions: The effects of fixed-dose combination therapy on all-cause mortality or ASCVD events are uncertain. A limited number of trials reported these outcomes, and the included trials were primarily designed to observe changes in ASCVD risk factor levels rather than clinical events, which may partially explain the observed differences in risk factors that were not translated into differences in clinical outcomes among the included trials. Fixed-dose combination therapy is associated with modest increases in adverse events compared with placebo, active comparator, or usual care but may be associated with improved adherence to a multidrug regimen. Ongoing, longer-term trials of fixed-dose combination therapy will help demonstrate whether short-term changes in risk factors might be maintained and lead to expected differences in clinical events based on these changes.
Conflict of interest statement
Mark Huffman has received grant support from Cochrane to support the production of this update. Dr. Huffman also receives grant support from World Heart Federation to serve as senior program advisor for its Emerging Leaders program, which is supported by Boehringer Ingelheim and Novartis and has been supported by AstraZeneca and Bupa. Dr. Huffman has also received travel support from the World Heart Federation for its polypill satellite meeting at the World Congress of Cardiology and Cardiovascular Health in 2016.
Figures







































Update of
-
Fixed-dose combination therapy for the prevention of cardiovascular disease.Cochrane Database Syst Rev. 2014 Apr 16;4(4):CD009868. doi: 10.1002/14651858.CD009868.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Mar 06;3:CD009868. doi: 10.1002/14651858.CD009868.pub3. PMID: 24737108 Free PMC article. Updated.
References
References to studies included in this review
CRUCIAL 2011 {published data only}
-
- Cho EJ, Kim JH, Sutradhar S, Yunis C, Westergaard M. Proactive multifactorial intervention strategy reduces the risk of cardiovascular disease estimated with region‐specific risk assessment models in Pacific Asian patients participating in the CRUCIAL trial. Journal of Korean Medical Science 2013;28:1741‐8. - PMC - PubMed
-
- Cho EJ, Kim JH, Sutradhar S, Yunis C, Westergaard M. Reduction in cardiovascular risk using a proactive multifactorial intervention is consistent among patients residing in Pacific Asian and non‐Pacific Asian regions: a CRUCIAL trial subanalysis. Vascular Health and Risk Management 2014;10:145‐56. - PMC - PubMed
-
- Hradec J, Zamorano J, Sutradhar S. Post hoc analysis of the Cluster Randomized Usual Care versus Caduet Investigation Assessing Long‐term risk (CRUCIAL) trial. Current Medical Research and Opinion 2013;29:589‐96. - PubMed
-
- Kim JH, Zamorano J, Erdine S, Pavia A, Al‐Khadra A, Sutradhar S, Yunis C. Reduction in cardiovascular risk using proactive multifactorial intervention versus usual care in younger (< 65 years) and older (≥ 65 years) patients in the CRUCIAL trial. Current Medical Research and Opinion 2013;29:453‐63. - PubMed
-
- Pavia A, Zamorano J, Sutradhar S, Yunis C. Changes in calculated coronary heart disease risk using proactive multifactorial intervention versus continued usual care in Latin‐American and non‐Latin‐American patients enrolled in the CRUCIAL trial. Current Medical Research and Opinion 2012;28:1667‐76. - PubMed
CUSP 2009 {published data only}
-
- Neutel JM, Bestermann WH, Dyess EM, Graff A, Kursun A, Sutradhar S, et al. The use of a single‐pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized, placebo‐controlled, multicenter study. Journal of Clinical Hypertension 2009;11(1):22‐30. - PMC - PubMed
FOCUS 2014 {published data only}
-
- Sanz G, Fuster V, Guzmán L, Guglietta A, Arnáiz JA, Martínez F, et al. The fixed‐dose combination drug for secondary cardiovascular prevention project: improving equitable access and adherence to secondary cardiovascular prevention with a fixed‐dose combination drug. Study design and objectives. American Heart Journal 2011;165:811‐7. - PubMed
IMPACT 2014 {published data only}
-
- Selak V, Elley C, Crengle S, Wadham A, Rafter N, Bullen C. Polypills for high risk patients: results of a New Zealand randomised controlled trial. Heart Lung and Circulation. 2014; Vol. 23:e13.
-
- Selak V, Elley CR, Bullen C, Crengle S, Wadham A, Rafter N, et al. Effect of fixed dose combination treatment on adherence and risk factor control among patients at high risk of cardiovascular disease: randomised controlled trial in primary care. BMJ 2014;348:g3318. - PubMed
-
- Selak V, Elley CR, Crengle S, Harwood M, Doughty R, Arroll B, et al. IMProving Adherence using Combination Therapy (IMPACT): design and protocol of a randomised controlled trial in primary care. Contemporary Clinical Trials 2011;32:909‐15. - PubMed
-
- Selak V, Harwood M, Raina EC, Bullen C, Wadham A, Parag V, et al. Polypill‐based therapy likely to reduce ethnic inequities in use of cardiovascular preventive medications: findings from a pragmatic randomised controlled trial. European Journal of Preventive Cardiology 2016;23:1537‐45. - PubMed
Kanyini GAP 2014 {published data only}
-
- Laba TL, Hayes A, Lo S, Peiris DP, Usherwood T, Hillis GS, et al. An economic case for a cardiovascular polypill? A cost analysis of the Kanyini GAP trial. Medical Journal of Australia 2014;201:671‐3. - PubMed
-
- Laba TL, Howard K, Rose J, Peiris D, Redfern J, Usherwood T, et al. Patient preferences for a polypill for the prevention of cardiovascular diseases. Annals of Pharmacotherapy 2015;49:528‐39. - PubMed
-
- Liu H, Laba TL, Massi L, Jan S, Usherwood T, Patel A, et al. Facilitators and barriers to implementation of a pragmatic clinical trial in Aboriginal health services. Medical Journal of Australia 2015;203:24‐7. - PubMed
-
- Liu H, Massi L, Laba TL, Peiris D, Usherwood T, Patel A, et al. Patients' and providers' perspectives of a polypill strategy to improve cardiovascular prevention in Australian primary health care: a qualitative study set within a pragmatic randomized, controlled trial. Circulation: Cardiovascular Quality and Outcomes 2015;8:301‐8. - PubMed
-
- Liu H, Patel A, Brown A, Eades S, Hayman N, Jan S, et al. Rationale and design of the Kanyini guidelines adherence with the polypill (Kanyini‐GAP) study: a randomised controlled trial of a polypill‐based strategy amongst indigenous and non indigenous people at high cardiovascular risk. BMC Public Health 2010;10:458. - PMC - PubMed
Malekzadeh 2010 {published data only}
-
- Malekzadeh F, Marshall T, Pourshams A, Gharravi M, Aslani A, Nateghi A, et al. A pilot double‐blind randomised placebo‐controlled trial of the effects of fixed‐dose combination therapy ('polypill') on cardiovascular risk factors. International Journal of Clinical Practice 2010;64(9):1220‐7. - PubMed
OLSTA 2016 {published data only}
-
- Park JS, Shin JH, Hong TJ, Seo HS, Shim WJ, Baek SH, et al. Efficacy and safety of fixed‐dose combination therapy with olmesartan medoxomil and rosuvastatin in Korean patients with mild to moderate hypertension and dyslipidemia: an 8‐week, multicenter, randomized, double‐blind, factorial‐design study (OLSTA‐D RCT: OLmesartan rosuvaSTAtin from Daewoong). Drug Design, Development and Therapy 2016;10:2599‐609. - PMC - PubMed
PILL 2011 {published and unpublished data}
-
- Lafeber M, Webster R, Visseren FLj, Bots ML, Grobbee DE, Spiering W, et al. Estimated cardiovascular relative risk reduction from fixed‐dose combination pill (polypill) treatment in a wide range of patients with a moderate risk of cardiovascular disease. European Journal of Preventive Cardiology 2016;23:1289‐97. - PubMed
Soliman 2009 {published data only}
TIPS 2009 {published data only}
-
- Indian Polycap Study, Yusuf S, Pais P, Afzal R, Xavier D, Teo K, et al. Effects of a polypill (Polycap) on risk factors in middle‐aged individuals without cardiovascular disease (TIPS): a phase II, double‐blind, randomised trial. Lancet 2009;373(9672):1341‐51. - PubMed
TOGETHER 2010 {published data only}
-
- Grimm R, Malik M, Yunis C, Sutradhar S, Kursun A, TOGETHER Investigators. Simultaneous treatment to attain blood pressure and lipid goals and reduced CV risk burden using amlodipine/atorvastatin single‐pill therapy in treated hypertensive participants in a randomized controlled trial. Vascular Health & Risk Management 2010;6:261‐71. - PMC - PubMed
-
- Grimm RH, Malik M, Yunis C, Sutradhar S, Kursun A. The critical importance of treating absolute cardiovascular risk as opposed to individual cardiovascular risk factors in isolation. Journal of Clinical Hypertension 2009;Conference:A120‐1.
UMPIRE 2013 {published data only}
-
- Salam A, Stewart F, Singh K, Thom S, Williams HJ, Patel A, et al. INterpreting the Processes of the UMPIRE Trial (INPUT): protocol for a qualitative process evaluation study of a fixed‐dose combination (FDC) strategy to improve adherence to cardiovascular medications. BMJ Open 2013;3:e002313. - PMC - PubMed
-
- Thom S, Field J, Poulter N, Patel A, Prabhakaran D, Stanton A, et al. Use of a Multidrug Pill In Reducing cardiovascular Events (UMPIRE): rationale and design of a randomised controlled trial of a cardiovascular preventive polypill‐based strategy in India and Europe. European Journal of Preventive Cardiology 2014;21(2):252‐61. [DOI: ] - PubMed
References to studies excluded from this review
Abdellatif 2012 {published data only}
Agabiti Rosei 2014 {published data only}
-
- Agabiti‐Rosei E, Manolis A, Zava D, Omboni S, Zodiac Study Group. Zofenopril plus hydrochlorothiazide and irbesartan plus hydrochlorothiazide in previously treated and uncontrolled diabetic and non‐diabetic essential hypertensive patients. Advances in Therapy 2014;31(2):217‐33. [DOI: ] - PMC - PubMed
Agarwal 2013 {published data only}
-
- Agarwal R, Weir MR. Blood pressure response with fixed‐dose combination therapy: comparing hydrochlorothiazide with amlodipine through individual‐level meta‐analysis. Journal of Hypertension 2013;31(8):1692‐701. [DOI: ] - PubMed
Anonymous 2010 {published data only}
-
- Anonymous. I take medications for blood pressure, cholesterol and other conditions. I have a two‐part question: are there any long‐term problems from taking a dozen or so pills a day, and has there been much progress in developing a "polypill" that combines several drugs into a single pill?. Heart Adviser 2010;13:8. - PubMed
Anonymous 2011 {published data only}
-
- Anonymous. Doubts surround study results touting polypill's benefits. The experimental pill contains four heart medications. Heart Adviser 2011;14:4. - PubMed
Anonymous 2012a {published data only}
-
- Anonymous. Treating many conditions‐‐with just one pill. The polypill could make your medicines much easier to take. Harvard Women's Health Watch 2012;20:5. - PubMed
Anonymous 2012b {published data only}
-
- Anonymous. Hypertension: improved therapy adherence after switch to a fixed drug combinations. MMW Fortschritte der Medizin 2012;154:78‐9. - PubMed
Anonymous 2013a {published data only}
-
- Anonymous. Coming soon: many drugs in one pill. Multidrug combinations may make medicines easier to swallow. Harvard Heart Letter 2013;23(7):5. - PubMed
Anonymous 2013b {published data only}
-
- Anonymous. Combined heart protection for the hypertensive patient. MMW Fortschritte der Medizin 2013;155(1):66‐7. - PubMed
Athyros 2013 {published data only}
-
- Athyros VG, Katsiki N, Karagiannis A. Cardiovascular risk reduction with combination of anti‐atherosclerotic medications in younger and older patients. Current Medical Research and Opinion 2013;29(7):791‐2. [DOI: ] - PubMed
Athyros 2014 {published data only}
-
- Athyros VG, Katsiki N, Karagiannis A, Mikhailidi DP. Combination of statin plus renin angiotensin system inhibition for the prevention or the treatment of atherosclerotic cardiovascular disease. Current Pharmaceutical Design 2014;20(40):6299‐305. - PubMed
Bashir 2011 {published data only}
-
- Bashir S, Sherwani MU, Shabbir I, Batool A. Efficacy of fix dose combination (atorvastatin and amlodipine) in treatment of uncontrolled hypertension and dyslipidemia. Journal of Ayub Medical College Abbottabad 2011;23:97‐100. - PubMed
Becerra 2015 {published data only}
Bittencourt 2013 {published data only}
-
- Bittencourt MS, Blaha M, Blankstein R, Vargas J, Budoff M, Agatston A, et al. Eligibility for polypill therapy, subclinical atherosclerosis, and cardiovascular events‐national implications for the appropriate use of preventive pharmacotherapy: multi‐ethnic study of atherosclerosis (MESA). Journal of the American College of Cardiology 2013;1:E813. [DOI: ] - PMC - PubMed
Bittencourt 2014 {published data only}
-
- Bittencourt MS, Blaha MJ, Blankstein R, Budoff M, Vargas JD, Blumenthal RS, et al. Polypill therapy, subclinical atherosclerosis, and cardiovascular events ‐ implications for the use of preventive pharmacotherapy. Journal of the American College of Cardiology 2014;63(5):434‐43. [DOI: 10.1016/j.jacc.2013.08.1640] - DOI - PMC - PubMed
Blank 2007 {published data only}
-
- Blank R, Hobbs FDR, Zamorano J, Girerd X. A single‐pill combination of amlodipine besylate and atorvastatin calcium (update). Drugs of Today 2007;43:157‐77. [DOI: ] - PubMed
Briasoulis 2013 {published data only}
-
- Briasoulis A, Bakris G. Initial single‐pill combination therapy for cardiovascular risk factor management: it is not just convenience. Journal of Hypertension 2013;31(8):1537‐8. [DOI: ] - PubMed
Bryant 2013 {published data only}
-
- Bryant L, Martini N, Chan J, Chang L, Marmoush A, Robinson B, et al. Could the polypill improve adherence? The patient perspective. Journal of Primary Health Care 2013;5(1):28‐35. - PubMed
Carey 2012 {published data only}
-
- Carey KM, Comee MR, Donovan JL, Kanaan AO. A polypill for all? Critical review of the polypill literature for primary prevention of cardiovascular disease and stroke. Annals of Pharmacotherapy 2012;46:688‐95. [DOI: ] - PubMed
Cass 2013 {published data only}
-
- Cass A, Patel A, Rodgers A. A pragmatic trial of a polypill‐based strategy to improve adherence to indicated preventive treatments among people at high cardiovascular disease risk. Nephrology 2013;18:33. [DOI: ] - PubMed
Castellano 2014a {published data only}
-
- Castellano JM, Sanz G, Fuster V. Evolution of the polypill concept and ongoing clinical trials. Canadian Journal of Cardiology 2014;30(5):520‐6. [DOI: ] - PubMed
Castellano 2014b {published data only}
Castellano 2015 {published data only}
-
- Castellano JM, Bueno H, Fuster V. The cardiovascular polypill: clinical data and ongoing studies. International Journal of Cardiology 2015;201:S8‐14. [DOI: ] - PubMed
Chae 2015 {published data only}
-
- Chae DW, Son M, Kim Y, Son H, Jang SB, Seo JM, et al. Pharmacokinetics of a telmisartan/rosuvastatin fixed‐dose combination: a single‐dose, randomized, open‐label, 2‐period crossover study in healthy Korean subjects. International Journal of Clinical Pharmacology and Therapeutics 2015;53(10):883‐9. [DOI: ] - PubMed
ChineseExpert 2013 {published data only}
-
- Chinese Expert, Consensus, Recommendation Group on the Development of Cardiovascular Disease Polypill in China. Consensus recommendation on the development of cardiovascular disease polypill in China. Chung Hua Hsin Hsueh Kuan Ping Tsa Chih 2013;41(2):91‐3. - PubMed
Chrysant 2014 {published data only}
Crunkhorn 2012 {published data only}
-
- Crunkhorn S. Trial watch: dual‐acting combination meets heart failure end point. Nature Reviews Drug Discovery 2012;11:740. [DOI: ] - PubMed
Dabhadkar 2013 {published data only}
deCates 2014 {published data only}
Delgado Montero 2012 {published data only}
Dimitrov 2012 {published data only}
-
- Dimitrov Y, Baguet JP, Hottelart C, Marboeuf P, Tartiere JM, Ducher M, et al. Is there a BP benefit of changing the time of aspirin administration in treated hypertensive patients?. European Journal of Preventive Cardiology 2012;19:706‐11. [DOI: ] - PubMed
Dresser 2012 {published data only}
-
- Dresser G, Nelson S, Mahon J, Zou G, Vandervoort M, Wong C, et al. Single pill combination therapy for treatment of hypertension in the combined stitch and STITCH2 study populations. Journal of Clinical Hypertension 2012; Vol. 14.
Dresser 2013 {published data only}
-
- Dresser GK, Nelson SA, Mahon JL, Zou G, Vandervoort MK, Wong CJ, et al. Simplified therapeutic intervention to control hypertension and hypercholesterolemia: a cluster randomized controlled trial (STITCH2). Journal of Hypertension 2013;31(8):1702‐13. [DOI: ] - PubMed
Elley 2012 {published data only}
Fedacko 2013 {published data only}
-
- Fedacko J, Pella D, Jarcuska P, Sabol F, Kmec J, Lopuchovsky T, et al. Slovak trial on cardiovascular risk reduction following national guidelines with CaDUET (the STRONG DUET study). Advances in Therapy 2013;30(1):60‐70. [DOI: ] - PubMed
Feldman 2012 {published data only}
-
- Feldman RD, Flack J, Howes L, Jenssen T, Reeves R, Shi H, et al. Impact of age and gender on blood pressure and low‐density lipoprotein cholesterol reduction: results of a pooled analysis. Current Medical Research and Opinion 2012;28:1421‐33. - PubMed
Feldman 2014 {published data only}
-
- Feldman RD, Nattel S. Increasing appreciation for the role of single‐pill combinations for the prevention of atherosclerotic disease: a pro‐polypill polemic. Canadian Journal of Cardiology 2014;30(5):517‐9. [DOI: ] - PubMed
Feng 2012 {published data only}
-
- Feng Y, Xu H, Chen K. Natural polypill Xuezhikang: its clinical benefit and potential multicomponent synergistic mechanisms of action in cardiovascular disease and other chronic conditions. Journal of Alternative and Complementary Medicine 2012;18:318‐28. [DOI: ] - PubMed
Galindo Ocana 2012 {published data only}
-
- Galindo‐Ocana J, Bernabeu‐Wittel M, Formiga F, Fuertes‐Martin A, Baron‐Franco B, Murcia‐Zaragoza JM, et al. Effects of renin‐angiotensin blockers/inhibitors and statins on mortality and functional impairment in polypathological patients. European Journal of Internal Medicine 2012;23:179‐84. [DOI: 10.1016/j.ejim.2011.06.004] - DOI - PubMed
Gaziano 2013 {published data only}
-
- Gaziano JM. Progress with the polypill?. JAMA 2013;310(9):910‐1. [DOI: ] - PubMed
Holzgreve 2014 {published data only}
-
- Holzgreve H. Health and longevity by one tablet daily. Fiction and hard facts on the polypill. MMW Fortschritte der Medizin 2014;156(1):55‐7. - PubMed
Huang 2016 {published data only}
-
- Huang Z, Chen C, Li S, Kong F, Shan P, Huang W. Combined treatment with amlodipine and atorvastatin calcium reduces circulating levels of intercellular adhesion molecule‐1 and tumor necrosis factor‐alpha in hypertensive patients with prediabetes. Frontiers in Aging Neuroscience 2016;8:206. [DOI: ] - PMC - PubMed
Huffman 2012 {published data only}
Huffman 2014 {published data only}
-
- Huffman MD, Yusuf S. Polypills: essential medicines for cardiovascular disease secondary prevention?. Journal of the American College of Cardiology 2014;63(14):1368‐70. [DOI: ] - PubMed
Ito 2012 {published data only}
Ivanovic 2013 {published data only}
-
- Ivanovic B, Tadic M. Fixed combination of amlodipine/atorvastatin: from mechanisms to trials. Journal of Cardiovascular Pharmacology and Therapeutics 2013;18(6):544‐9. [DOI: ] - PubMed
Jadhav 2014 {published data only}
-
- Jadhav U, Hiremath J, Namjoshi DJ, Gujral VK, Tripathi KK, Siraj M, et al. Blood pressure control with a single‐pill combination of indapamide sustained‐release and amlodipine in patients with hypertension: The EFFICIENT Study. PLoS One 2014;9(4):6. [DOI: 10.1371/journal.pone.0092955] - DOI - PMC - PubMed
Jang 2015 {published data only}
Jaques 2011 {published data only}
-
- Jaques H. The polypill concept: the story so far. [Erratum appears in European Heart Journal 2012 Mar; 33(5):551]. European Heart Journal 2011;32:2471‐2. - PubMed
Kawashiri 2015 {published data only}
-
- Kawashiri MA, Sakata K, Gamou T, Kanaya H, Miwa K, Ueda K, et al. Impact of combined lipid lowering with blood pressure control on coronary plaque regression: rationale and design of MILLION study. Heart Vessels 2015;30(5):580‐6. [DOI: ] - PubMed
Kereiakes 2012 {published data only}
-
- Kereiakes DJ, Chrysant SG, Izzo JL, Littlejohn T, Melino M, Lee J, et al. Olmesartan/amlodipine/hydrochlorothiazide in participants with hypertension and diabetes, chronic kidney disease, or chronic cardiovascular disease: a subanalysis of the multicenter, randomized, double‐blind, parallel‐group TRINITY study. Cardiovascular Diabetology 2012;11:13. [DOI: 10.1186/1475-2840-11-134] - DOI - PMC - PubMed
Khaled 2015 {published data only}
Laba 2014a {published data only}
-
- Laba TL, Howard K, Rose J, Jan S. Using DCE to assess adherence and treatment preferences for combination therapies for cardiovascular disease. Global Heart 2014;1:e44. [DOI: ]
Laba 2014b {published data only}
-
- Laba TL, Hayes A, Jan S, Rodgers A, Patel A, Cass A, et al. Can a CVD polypill save money in the 'real world'?. Value in Health 2014;17(7):A482. [DOI: ] - PubMed
Lafeber 2011 {published data only}
-
- Lafeber M, Spiering W, Bots ML, Valk V, Visseren FL, Grobbee DE. Cardiovascular polypill in high risk patients. Nederlands Tijdschrift Voor Geneeskunde 2011;155:A3070. - PubMed
Lafeber 2012 {published data only}
-
- Lafeber M, Spiering W, Singh K, Guggilla RK, Patil V, Webster R. The cardiovascular polypill in high‐risk patients. European Journal of Preventive Cardiology 2012;19:1234‐42. [DOI: ] - PubMed
Lafeber 2013a {published data only}
-
- Lafeber M, Grobbee DE, Spiering W, Graaf Y, Bots ML, Visseren FL. The combined use of aspirin, a statin, and blood pressure‐lowering agents (polypill components) in clinical practice in patients with vascular diseases or type 2 diabetes mellitus. European Journal of Preventive Cardiology 2013;20(5):771‐8. [DOI: ] - PubMed
Lafeber 2013b {published data only}
-
- Lafeber M, Spiering W, Graaf Y, Nathoe H, Bots ML, Grobbee DE, et al. The combined use of aspirin, a statin, and blood pressure‐lowering agents (polypill components) and the risk of vascular morbidity and mortality in patients with coronary artery disease. American Heart Journal 2013;166(2):282‐9.e1. [DOI: ] - PubMed
Lafeber 2014a {published data only}
-
- Lafeber M, Grobbee DE, Bots ML, Thom S, Webster R, Rodgers A, et al. The Evening versus Morning Polypill Utilization Study: The TEMPUS rationale and design. European Journal of Preventive Cardiology 2014;21(4):425‐33. [DOI: ] - PubMed
Lafeber 2014b {published data only}
-
- Lafeber M, Spiering W, Grobbee DE, Bots ML, Webster R, Thom S, et al. Impact of switching from different treatment regimens to a fixed‐dose combination pill (polypill) in patients with cardiovascular disease or similarly high risk. Circulation 2014;129:AP381. - PubMed
Lafeber 2014c {published data only}
-
- Lafeber M, Stanton A, Thom S, Hughes A, Grobbee DE, Rodgers A, et al. A randomized controlled trial of a cardiovascular fixed‐dose combination pill (polypill) treatment strategy compared with usual care on carotid intima media thickness progression in individuals at high risk of cardiovascular disease. Circulation 2014;129:AP445.
Lafeber 2014d {published data only}
-
- Lafeber M, Grobbee DE, Schrover IM, Thom S, Webster R, Rodgers A, et al. The effect of morning or evening use of a fixed‐dose combination pill (polypill) on LDL‐cholesterol, ambulatory blood pressure and adherence in patients with cardiovascular disease. European Journal of Preventive Cardiology 2014;1:S132. [DOI: ]
Lafeber 2015 {published data only}
-
- Lafeber M, Grobbee DE, Schrover IM, Thom S, Webster R, Rodgers A, et al. Comparison of a morning polypill, evening polypill and individual pills on LDL‐cholesterol, ambulatory blood pressure and adherence in high‐risk patients; a randomized crossover trial. International Journal of Cardiology 2015;181:193‐9. [DOI: ] - PubMed
Lafeber 2016 {published data only}
Law 2006 {published data only}
Liu 2014 {published data only}
-
- Liu H, Massi L, Laba T, Jan S. Understanding adherence to a cardiovascular polypill strategy‐a process evaluation of a pragmatic clinical trial. Global Heart 2014;1:e118. [DOI: ]
Liu 2015 {published data only}
-
- Liu H, Massi L, Laba TL, Peiris D, Usherwood T, Patel A, et al. Patients' and providers' perspectives of a polypill strategy to improve cardiovascular prevention in Australian Primary Health Care. Circulation: Cardiovascular Quality and Outcomes 2015;8(3):301‐8. [DOI: ] - PubMed
Marazzi 2016 {published data only}
-
- Marazzi G, Pelliccia F, Campolongo G, Cacciotti L, Poggi S, Tanzilli A, et al. Greater cardiovascular risk reduction with once‐daily fixed combination of three antihypertensive agents and statin versus free‐drug combination: The ALL‐IN‐ONE trial. International Journal of Cardiology 2016;222:885‐7. [DOI: ] - PubMed
Mishchenko 2014 {published data only}
-
- Mishchenko O, Bezditko N, Adonkina V, Tkachova O. Pharmacoeconomic grounding of using polypill amlodipine with atorvastatin versus monodrugs in patients with hypertension and dyslipidemia in Ukraine. Value in Health 2014;17(7):A475. [DOI: ] - PubMed
Mossello 2015 {published data only}
-
- Mossello E. Targeting vascular risk factors in older adults: from polypill to personalized prevention. JAMA Internal Medicine 2015;175(12):1949‐50. [DOI: ] - PubMed
Neutel 2009 {published data only}
-
- Neutel JM, Bestermann WH, Dyess EM, Graff A, Kursun A, Sutradhar S, et al. The use of a single‐pill calcium channel blocker/statin combination in the management of hypertension and dyslipidemia: a randomized, placebo‐controlled, multicenter study. Journal of Clinical Hypertension 2009;11(1):22‐30. [DOI: 10.1111/j.1751-7176.2008.00058.x] - DOI - PMC - PubMed
Nguyen 2013 {published data only}
OliverasVila 2014 {published data only}
-
- Oliveras Vila T, Ferrer Massot M, Curos Abadal A, Rueda Sobella F, Serra Flores J, Carrillo Suarez, et al. Real‐life use of the polypill components (ASA+ACEI+statins) after an acute coronary syndrome and long‐term mortality. International Journal of Cardiology 2014;177(1):209‐10. [DOI: ] - PubMed
Reiner 2013 {published data only}
Selak 2013 {published data only}
Selak 2016 {published data only}
-
- Selak V, Bullen C, Stepien S, Arroll B, Bots M, Bramley D, et al. Do polypills lead to neglect of lifestyle risk factors? Findings from an individual participant data meta‐analysis among 3140 patients at high risk of cardiovascular disease. European Journal of Preventive Cardiology 2016;23(13):1393‐400. [DOI: ] - PubMed
Sepanlou 2012 {published data only}
-
- Sepanlou SG, Farzadfar F, Jafari E, Danaei G. Cardiovascular disease prevention using fixed dose pharmacotherapy in Iran: updated meta‐analyses and mortality estimation. Archives of Iranian Medicine 2012;15(9):531‐7. [DOI: ] - PubMed
Sigamani 2012 {published data only}
-
- Sigamani A, Pais P, Xavier D, Koon T, Xavier F, Girish P, et al. Comparison of risk factor reduction and tolerability of a full dose versus low dose of a polypill (polycap) in individuals at high risk of cardiovascular diseases: a phase II, double blind randomized trial. Circulation 2012;125(19):e803. [DOI: ] - PubMed
Simonyi 2016 {published data only}
-
- Simonyi G, Ferenci T. One year persistence of atorvastatin and amlodipine fixed dose combination versus atorvastatin therapy. Orvosi Hetilap 2016;157(11):425‐9. [DOI: ] - PubMed
Son 2013 {published data only}
-
- Son H, Roh H, Lee D, Chang H, Kim J, Yun C, et al. Pharmacokinetics of rosuvastatin/olmesartan fixed‐dose combination: a single‐dose, randomized, open‐label, 2‐period crossover study in healthy Korean subjects. Clinical Therapeutics 2013;35(7):915‐22. [DOI: ] - PubMed
Tanaka 2014 {published data only}
-
- Tanaka M, Nishimura R, Nishimura T, Kawai T, Meguro S, Irie J, et al. Effect of single tablet of fixed‐dose amlodipine and atorvastatin on blood pressure/lipid control, oxidative stress, and medication adherence in type 2 diabetic patients. Diabetology and Metabolic Syndrome 2014;6:8. [DOI: 10.1186/1758-5996-6-56] - DOI - PMC - PubMed
Truelove 2014 {published data only}
-
- Truelove M, Webster R, Bompoint S, Patel A. Impact of pill burden on the effects of a polypill‐based strategy on use of indicated medications in people with or at high risk of cardiovascular disease. Global Heart 2014;1:e298. [DOI: ]
Wald 2016 {published data only}
Wang 2012 {published data only}
-
- Wang YQ, Hu Z, Yang Y, Gao PJ. Effect of amlodipine and amlodipine plus atorvastatin on impaired vascular function in patients with hypertension. Journal of Hypertension 2012;30:e163. [DOI: ]
Webster 2013 {published data only}
-
- Webster R, Patel A, Billot L, Cass, A, Burch C, Neal B, et al. Prospective meta‐analysis of trials comparing fixed dose combination based care with usual care in individuals at high cardiovascular risk: the SPACE Collaboration. International Journal of Cardiology 2013;170(1):30‐5. [DOI: ] - PubMed
Webster 2014 {published data only}
Webster 2015a {published data only}
-
- Webster R, Rodgers A. Polypill: progress and challenges to global use: update on the trials and policy implementation. Current Cardiology Reports 2015;17(12):121. [DOI: ] - PubMed
Webster 2015b {published data only}
-
- Webster R, Bullen C, Patel A, Rodgers A, Selak V, Thom S. Impact of cardiovascular polypill based therapy on healthy lifestyle behavior. European Journal of Preventive Cardiology 2015;1:S150. [DOI: ]
Webster 2016a {published data only}
-
- Webster R, Patel A, Selak V, Billot L, Bots ML, Brown A, et al. Effectiveness of fixed dose combination medication ('polypills') compared with usual care in patients with cardiovascular disease or at high risk: a prospective, individual patient data meta‐analysis of 3140 patients in six countries. International Journal of Cardiology 2016;205:147‐56. [DOI: ] - PubMed
Webster 2016b {published data only}
-
- Webster R, Rodgers A. Polypill treatments for cardiovascular diseases. Expert Opinion on Drug Delivery 2016;13(1):1‐6. [DOI: ] - PubMed
Wei 2013 {published data only}
Wijns 2014 {published data only}
Wiley 2014 {published data only}
Xing 2013 {published data only}
-
- Xing DM, Zhang JH, Li L, Zhu MJ, Shang HC. Intervention effects and safety of cardiovascular polypill for the relevant risk factors of coronary heart disease: a systematic review. Chinese Journal of Evidence‐Based Medicine 2013;13:446‐51. [DOI: ]
Zeng 2016 {published data only}
Zomer 2013 {published data only}
-
- Zomer E, Owen A, Magliano DJ, Ademi Z, Reid CM, Liew D. Predicting the impact of polypill use in a metabolic syndrome population: an effectiveness and cost‐effectiveness analysis. American Journal of Cardiovascular Drugs 2013;13(2):121‐8. [DOI: ] - PubMed
References to studies awaiting assessment
Fommei 2015 {published data only}
-
- Fommei E, Ghione S, Biagini S, Corrao G, Mancia G. A proposal for the idea of a flexible‐combination polypill in arterial hypertension. Journal of Hypertension. 2015; Vol. 33:e258.
NCT00530946 {published data only}
-
- NCT00530946. A randomized study to evaluate efficacy and safety of a fixed combination therapy of amlodipine and atorvastatin [A multi‐center, randomized study to evaluate efficacy and safety of a fixed combination therapy of amlodipine and atorvastatin in the treatment of concurrent hypertension and hyper‐LDL‐cholesterolemia]. clinicaltrials.gov/ct2/show/NCT00530946 (first received 13 September 2007).
NCT01004705 {published data only}
-
- NCT01004705. Cardiovascular fixed dose combination pill: a pharmacodynamic study of a fixed dose combination of acetylsalicylic acid, simvastatin, and ramipril in subjects with elevated LDL cholesterol. clinicaltrials.gov/ct2/show/NCT01004705 (first received 23 October 2009).
NCT01005290 {published data only}
-
- NCT01005290. Cardiovascular fixed dose combination pill: a pharmacodynamic interaction study to evaluate the effect of a fixed dose combination of acetylsalicylic acid, simvastatin and ramipril (cardiovascular fixed dose combination pill) on blood pressure. clinicaltrials.gov/ct2/show/NCT01005290 (first received 22 October 2009).
NCT01362218 {published data only}
-
- NCT01362218. Cardiovascular fixed combination pill ASR: pharmacodynamic clinical trial of a fixed dose combination of acetylsalicylic acid, simvastatin, and ramipril (cardiovascular polypill) on LDL cholesterol. clinicaltrials.gov/ct2/show/NCT01362218 (first received 26 May 2011).
NCT01406431 {published data only}
-
- NCT01406431. A single dose, sequence‐randomized, open‐label, 2x2 crossover study to compare pharmacokinetics between pitavastatin and valsartan co‐administration and Livalo® fixed combination drug in healthy male subjects. clinicaltrials.gov/ct2/show/NCT01406431 (first received 26 July 2011).
NCT01764178 {published data only}
-
- NCT01764178. A single dose, sequence‐randomized, open‐label, 2x2 crossover study to compare pharmacokinetics between pitavastatn and valsartan co‐administration and Livalo complex product in healthy male subjects. clinicaltrials.gov/ct2/show/NCT01764178 (first received 1 January 2013).
NCT02075619 {published data only}
-
- NCT02075619. An open‐label, randomized, single dose, three‐way crossover, six sequence pilot study to evaluate the relative bioavailability of one amlodipine 10mg tablet and rosuvastatin 20mg tablet to two fixed dose combination tablet formulations of amlodipine (10mg) and rosuvastatin (20mg) in healthy adult male and female subjects under fasting conditions. clinicaltrials.gov/ct2/show/NCT02075619 (first received 27 February 2014).
NCT02569814 {published data only}
-
- NCT02569814. A study to compare the pharmacokinetics and safety of a fixed dose combination of fimasartan/amlodipine/rosuvastatin. clinicaltrials.gov/ct2/show/NCT02569814 (first received 5 October 2015).
NCT02662894 {published data only}
-
- NCT02662894. Efficacy of fixed‐dose combination of valsartan + rosuvastatin versus their isolated components for hypertension and dyslipidemia. clinicaltrials.gov/ct2/show/NCT02662894 (first received 23 November 2015).
NCT02791958 {published data only}
-
- NCT02791958. Pharmacodynamic equivalence study of ramipril 10 mg and atorvastatin 40 mg administered as a cardiovascular fixed dose combination pill AAR as compared to monotherapy with the reference products Altace® 10 mg and Lipitor® 40 mg. clinicaltrials.gov/ct2/show/NCT02791958 (first received 15 February 2016).
NCT02842359 {published data only}
-
- NCT02842359. Efficacy evaluation of metabolic, anti‐inflammatory, and antioxidative factors of irbesartan/atorvastatin fixed‐dose combination in type 2 diabetic patients diagnosed with hyperlipidemia and hypertension, with adequately controlled blood glucose levels. clinicaltrials.gov/ct2/show/NCT02842359 (first received 20 July 2016).
References to ongoing studies
INTEGRATE {published data only}
-
- Hayek A, Joshi R, Usherwood T, Webster R, Kaur B, Saini B, et al. An integrated general practice and pharmacy‐based intervention to promote the use of appropriate preventive medications among individuals at high cardiovascular disease risk: protocol for a cluster randomized controlled trial. Implementation Science 2016;11:129. - PMC - PubMed
-
- Joshi R, Patel A, Peiris D, Saini B, Usherwood T, Armor C, et al. INTegrated Electronic General practice support tool, phaRmacy led intervention And combination Therapy Evaluation trial (INTEGRATE). Heart Lung and Circulation. 2015:S385.
NCT01646437 {published data only}
-
- NCT01646437. The International Polycap Study‐3. clinicaltrials.gov/ct2/show/NCT01646437 (first received 10 July 2012).
NCT01826019 {published data only}
-
- NCT01826019. Heart Outcomes Prevention and Evaluation 4 (HOPE‐4). clinicaltrials.gov/ct2/show/NCT01826019 (first received 31 March 2013).
NCT02278471 {published data only}
-
- NCT02278471. The SCCS polypill pilot trial. clinicaltrials.gov/ct2/show/NCT02278471 (first received 13 October 2014).
NCT02596126 {published data only}
-
- NCT02596126. SEcondary prevention of Cardiovascular disease in the Elderly trial (SECURE). clinicaltrials.gov/ct2/show/NCT02596126 (first received 5 October 2015).
PolyIran {published data only}
-
- Merat S, Poustchi H, Hemming K, Jafari E, Radmard AR, Nateghi A, et al. PolyPill for prevention of cardiovascular disease in an urban Iranian population with special focus on nonalcoholic steatohepatitis: a pragmatic randomized controlled trial within a cohort (PolyIran ‐ Liver) ‐ study protocol. Archives of Iranian Medicine 2015;18:515‐23. - PubMed
-
- NCT01271985. Prevention of cardiovascular disease in middle‐aged and elderly Iranians using a single polypill (PolyIran). clinicaltrials.gov/ct2/show/NCT01271985 (first received 14 December 2010).
-
- Roshandel G, Ostovaneh MR, Poustchi H, Malekzadeh F, Sepanlou SG, Honarvar MR, et al. Reliability analysis of a newly developed questionnaire for quality control of follow‐up visits in PolyIran study. Archives of Iranian Medicine 2016;19:551‐5. - PubMed
Additional references
ALLHAT‐investigators 2002
-
- ALLHAT‐investigators. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288(23):2981‐97. - PubMed
Armitage 2010
-
- Armitage JM, Bowman L, Clarke RJ, Wallendszus K, Bulbulia R, Rahimi K, et al. Effects of homocysteine‐lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA 2010;303(24):2486‐94. - PubMed
ATT‐Collaboration 2002
Baigent 2005
-
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267‐78. - PubMed
Baigent 2009
Bangalore 2007
-
- Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed‐dose combinations improve medication compliance: a meta‐analysis. American Journal of Medicine 2007;120:713‐8. [PUBMED: 17679131] - PubMed
Beaglehole 2011
-
- Beaglehole R, Bonita R, Horton R, Adams C, Alleyne G, Asaria P, et al. Priority actions for the non‐communicable disease crisis. Lancet 2011;377(9775):1438‐47. - PubMed
Berry 2012
Colhoun 2004
-
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo‐controlled trial. Lancet 2004;364(9435):685‐96. - PubMed
Collins 1990
-
- Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, short‐term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 1990;335(8693):827‐38. - PubMed
CTT 2012
de Cates 2014
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Ebrahim 2011
Egger 1997
ESC 2016
-
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European Heart Journal 2016;37(29):2315‐81. - PMC - PubMed
Franco 2004
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. - PubMed
Gaede 2003
-
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. New England Journal of Medicine 2003;348(5):383‐93. - PubMed
GRADE 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Gupta 2010
-
- Gupta AK, Arshad S, Poulter NR. Compliance, safety, and effectiveness of fixed‐dose combinations of antihypertensive agents: a meta‐analysis. Hypertension 2010;55(2):399‐407. - PubMed
Higgins 2003
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holmes 2011
HPSCG 2002
-
- HPSCG. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet 2002;360(9326):7‐22. - PubMed
Julius 2004
-
- Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363(9426):2022‐31. - PubMed
Karmali 2016
Kearney 2008
-
- Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, et al. Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet 2008;371(9607):117‐25. - PubMed
LaRosa 2005
-
- LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. New England Journal of Medicine 2005;352(14):1425‐35. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of InterventionsVersion 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lonn 2010
-
- Lonn E, Bosch J, Teo KK, Pais P, Xavier D, Yusuf S. The polypill in the prevention of cardiovascular diseases: key concepts, current status, challenges, and future directions. Circulation 2010;122(20):2078‐88. - PubMed
Meader 2014
Messerli 2006
Moher 2009
Neaton 1992
-
- Neaton JD, Wentworth D. Serum cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Overall findings and differences by age for 316,099 white men. Multiple Risk Factor Intervention Trial Research Group. Archives of Internal Medicine 1992;152(1):56‐64. - PubMed
NICE 2008
-
- NICE. Lipid Modification. Cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. www.nice.org.uk/nicemedia/pdf/CG67NICEguideline.pdf 2008 (Accessed 17 January 2017).
NICE 2010
-
- NICE. Cardiovascular disease prevention. www.nice.org.uk/guidance/PH25 2010 (Accessed 17 January 2017).
O'Donnell 2010
-
- O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet 2010;376(9735):112‐23. - PubMed
Ostergren 2008
-
- Ostergren J, Poulter NR, Sever PS, Dahlof B, Wedel H, Beevers G, et al. The Anglo‐Scandinavian Cardiac Outcomes Trial: blood pressure‐lowering limb: effects in patients with type II diabetes. Journal of Hypertension 2008;26(11):2103‐11. - PubMed
Papademetriou 2003
-
- Papademetriou V, Piller LB, Ford CE, Gordon D, Hartney TJ, Geraci TS, et al. Characteristics and lipid distribution of a large, high‐risk, hypertensive population: the lipid‐lowering component of the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Journal of Clinical Hypertension 2003;5(6):377‐84. - PMC - PubMed
Perk 2012
-
- Perk J, DeBacker G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). European Journal of Cardiology 2012;33:1635‐701. [PUBMED: 10.1093/eurheartj/ehs092] - PubMed
PILL‐collaborative 2011
Preston 2007
-
- Preston RA, Harvey P, Herfert O, Dykstra G, Jukema JW, Sun F, et al. A randomized, placebo‐controlled trial to evaluate the efficacy, safety, and pharmacodynamic interaction of coadministered amlodipine and atorvastatin in 1660 patients with concomitant hypertension and dyslipidemia: the respond trial. Journal of Clinical Pharmacology 2007;47(12):1555‐69. - PubMed
Rashid 2003
-
- Rashid P, Leonardi‐Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke 2003;34(11):2741‐8. - PubMed
Roth 2015
Sever 2003
-
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower‐than‐average cholesterol concentrations, in the Anglo‐Scandinavian Cardiac Outcomes Trial ‐ Lipid Lowering Arm (ASCOT‐LLA): a multicentre randomised controlled trial. Lancet 2003;361(9364):1149‐58. - PubMed
Stone 2013
-
- Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013;129(25 Suppl 2):S1‐45. [DOI: ] - PubMed
Taylor 2013
Thomas 2002
-
- Thomas F, Bean K, Guize L, Quentzel S, Argyriadis P, Benetos A. Combined effects of systolic blood pressure and serum cholesterol on cardiovascular mortality in young. European Heart Journal 2002;23(7):528‐35. - PubMed
Turnbull 2003
-
- Turnbull F. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events: results of prospectively‐designed overviews of randomised trials. Lancet 2003;362(9395):1527‐35. - PubMed
Vartiainen 2010
-
- Vartiainen E, Laatikainen T, Peltonen M, Juolevi A, Mannisto S, Sundvall J, et al. Thirty‐five‐year trends in cardiovascular risk factors in Finland. International Journal of Epidemiology 2010;39(2):504‐18. - PubMed
Viera 2011
Virdee 2013
Wald 2003
Wald 2011
-
- Wald DS, Wald NJ. The polypill in the prevention of cardiovascular disease. Preventive Medicine 2011;52(1):16‐7. - PubMed
WHO 2001
-
- World Health Organization. Secondary prevention of non‐communicable disease in low and middle income countries through community‐based and health service interventions. Report of WHO‐Wellcome Trust meeting of experts (1‐3 August 2001). apps.who.int/iris/bitstream/10665/42567/1/WHO_MPN_CVD_2002.01.pdf 2001.
WHO 2013
-
- World Health Organization. Global action plan for the prevention and control of noncommunicable diseases, 2013‐202. apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf 2013;1:1‐55.
WHO 2016
-
- World Health Organization. HEARTS Technical package for cardiovascular disease management in primary health care. www.who.int/cardiovascular_diseases/hearts/Hearts_package.pdf 2016;1:1‐76.
Yusuf 2002
-
- Yusuf S. Two decades of progress in preventing vascular disease. Lancet 2002;360(9326):2‐3. - PubMed
Yusuf 2004
-
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet 2004;364(9438):937‐52. - PubMed
Yusuf 2011
-
- Yusuf S, Islam S, Chow CK, Rangarajan S, Dagenais G, Diaz R, et al. Use of secondary prevention drugs for cardiovascular disease in the community in high‐income, middle‐income, and low‐income countries (the PURE study): a prospective epidemiological survey. Lancet 2011;378(9798):1231‐43. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

