Neuregulin-1β induces proliferation, survival and paracrine signaling in normal human cardiac ventricular fibroblasts
- PMID: 28263756
- PMCID: PMC5715731
- DOI: 10.1016/j.yjmcc.2017.03.001
Neuregulin-1β induces proliferation, survival and paracrine signaling in normal human cardiac ventricular fibroblasts
Abstract
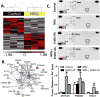
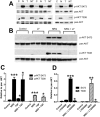
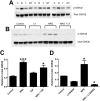
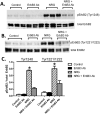
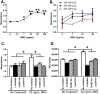
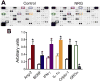
Neuregulin-1β (NRG-1β) is critical for cardiac development and repair, and recombinant forms are currently being assessed as possible therapeutics for systolic heart failure. We previously demonstrated that recombinant NRG-1β reduces cardiac fibrosis in an animal model of cardiac remodeling and heart failure, suggesting that there may be direct effects on cardiac fibroblasts. Here we show that NRG-1β receptors (ErbB2, ErbB3, and ErbB4) are expressed in normal human cardiac ventricular (NHCV) fibroblast cell lines. Treatment of NHCV fibroblasts with recombinant NRG-1β induced activation of the AKT pathway, which was phosphoinositide 3-kinase (PI3K)-dependent. Moreover, the NRG-1β-induced PI3K/AKT signaling in these cells required phosphorylation of both ErbB2 and ErbB3 receptors at tyrosine (Tyr)1248 and Tyr1289 respectively. RNASeq analysis of NRG-1β-treated cardiac fibroblasts obtained from three different individuals revealed a global gene expression signature consistent with cell growth and survival. We confirmed enhanced cellular proliferation and viability in NHCV fibroblasts in response to NRG-1β, which was abrogated by PI3K, ErbB2, and ErbB3 inhibitors. NRG-1β also induced production and secretion of cytokines (interleukin-1α and interferon-γ) and pro-reparative factors (angiopoietin-2, brain-derived neurotrophic factor, and crypto-1), suggesting a role in cardiac repair through the activation of paracrine signaling.
Keywords: Cardiac fibroblast; ErbB3; Neuregulin; RNASeq; Survival.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
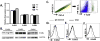
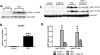

References
-
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. - PubMed
-
- Fuller SJ, Sivarajah K, Sugden PH. ErbB receptors, their ligands, and the consequences of their activation and inhibition in the myocardium. J. Mol. Cell. Cardiol. 2008;44:831–854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

