EGFR-mediated macrophage activation promotes colitis-associated tumorigenesis
- PMID: 28263971
- PMCID: PMC5501754
- DOI: 10.1038/onc.2017.23
EGFR-mediated macrophage activation promotes colitis-associated tumorigenesis
Abstract
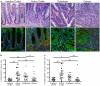
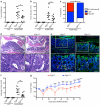
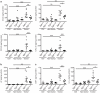
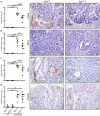
Epidermal growth factor receptor (EGFR) signaling is a known mediator of colorectal carcinogenesis. Studies have focused on the role of EGFR signaling in epithelial cells, although the exact nature of the role of EGFR in colorectal carcinogenesis remains a topic of debate. Here, we present evidence that EGFR signaling in myeloid cells, specifically macrophages, is critical for colon tumorigenesis in the azoxymethane-dextran sodium sulfate (AOM-DSS) model of colitis-associated carcinogenesis (CAC). In a human tissue microarray, colonic macrophages demonstrated robust EGFR activation in the pre-cancerous stages of colitis and dysplasia. Utilizing the AOM-DSS model, mice with a myeloid-specific deletion of Egfr had significantly decreased tumor multiplicity and burden, protection from high-grade dysplasia and significantly reduced colitis. Intriguingly, mice with gastrointestinal epithelial cell-specific Egfr deletion demonstrated no differences in tumorigenesis in the AOM-DSS model. The alterations in tumorigenesis in myeloid-specific Egfr knockout mice were accompanied by decreased macrophage, neutrophil and T-cell infiltration. Pro-tumorigenic M2 macrophage activation was diminished in myeloid-specific Egfr-deficient mice, as marked by decreased Arg1 and Il10 mRNA expression and decreased interleukin (IL)-4, IL10 and IL-13 protein levels. Surprisingly, diminished M1 macrophage activation was also detectable, as marked by significantly reduced Nos2 and Il1b mRNA levels and decreased interferon (IFN)-γ, tumor necrosis factor (TNF)-α and IL-1β protein levels. The alterations in M1 and M2 macrophage activation were confirmed in bone marrow-derived macrophages from mice with the myeloid-specific Egfr knockout. The combined effect of restrained M1 and M2 macrophage activation resulted in decreased production of pro-angiogenic factors, CXCL1 and vascular endothelial growth factor (VEGF), and reduced CD31+ blood vessels, which likely contributed to protection from tumorigenesis. These data reveal that EGFR signaling in macrophages, but not in colonic epithelial cells, has a significant role in CAC. EGFR signaling in macrophages may prove to be an effective biomarker of CAC or target for chemoprevention in patients with inflammatory bowel disease.
Figures


References
-
- Brower V. Feeding the flame: new research adds to role of inflammation in cancer development. J Natl Cancer Inst. 2005;97:251–253. - PubMed
-
- Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297–305. - PubMed
-
- Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–2114. e2105. - PubMed
MeSH terms
Substances
Grants and funding
- P50 CA095103/CA/NCI NIH HHS/United States
- R01 CA190612/CA/NCI NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R01 AT004821/AT/NCCIH NIH HHS/United States
- P01 CA116087/CA/NCI NIH HHS/United States
- F31 DK107159/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- R01 DK053620/DK/NIDDK NIH HHS/United States
- P01 CA028842/CA/NCI NIH HHS/United States
- I01 BX001453/BX/BLRD VA/United States
- IK2 BX002126/BX/BLRD VA/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- T32 GM008554/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

