GATA3 interacts with and stabilizes HIF-1α to enhance cancer cell invasiveness
- PMID: 28263977
- PMCID: PMC5537608
- DOI: 10.1038/onc.2017.8
GATA3 interacts with and stabilizes HIF-1α to enhance cancer cell invasiveness
Erratum in
-
GATA3 interacts with and stabilizes HIF-1α to enhance cancer cell invasiveness.Oncogene. 2017 Jul 27;36(30):4380. doi: 10.1038/onc.2017.196. Epub 2017 Jun 12. Oncogene. 2017. PMID: 28604747 Free PMC article.
Abstract
GATA binding protein 3 (GATA3) is indispensable in development of human organs. However, the role of GATA3 in cancers remains elusive. Hypoxia inducible factor (HIF)-1 plays an important role in pathogenesis of human cancers. Regulation of HIF-1α degradation is orchestrated through collaboration of its interacting proteins. In this study, we discover that GATA3 is upregulated in head and neck squamous cell carcinoma (HNSCC) and is an independent predictor for poor disease-free survival. GATA3 promotes invasive behaviours of HNSCC and melanoma cells in vitro and in immunodeficient mice. Mechanistically, GATA3 physically associates with HIF-1α under hypoxia to inhibit ubiquitination and proteasomal degradation of HIF-1α, which is independent of HIF-1α prolyl hydroxylation. Chromatin immunoprecipitation assays show that the GATA3/HIF-1α complex binds to and regulates HIF-1 target genes, which is also supported by the microarray analysis. Notably, the GATA3-mediated invasiveness can be significantly reversed by HIF-1α knockdown, suggesting a critical role of HIF-1α in the underlying mechanism of GATA3-mediated effects. Our findings suggest that GATA3 stabilizes HIF-1α to enhance cancer invasiveness under hypoxia and support the GATA3/HIF-1α axis as a potential therapeutic target for cancer treatment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
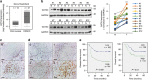
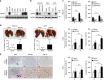


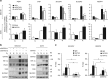
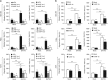
Similar articles
-
Dysregulation of hypoxia inducible factor-1alpha in head and neck squamous cell carcinoma cell lines correlates with invasive potential.Laryngoscope. 2004 Mar;114(3):418-23. doi: 10.1097/00005537-200403000-00006. Laryngoscope. 2004. PMID: 15091212
-
Overexpression and nuclear translocation of hypoxia-inducible factor prolyl hydroxylase PHD2 in head and neck squamous cell carcinoma is associated with tumor aggressiveness.Clin Cancer Res. 2006 Feb 15;12(4):1080-7. doi: 10.1158/1078-0432.CCR-05-2022. Clin Cancer Res. 2006. PMID: 16489060
-
Hypoxia promotes migration/invasion and glycolysis in head and neck squamous cell carcinoma via an HIF-1α-MTDH loop.Oncol Rep. 2017 Nov;38(5):2893-2900. doi: 10.3892/or.2017.5949. Epub 2017 Sep 7. Oncol Rep. 2017. PMID: 28901527
-
2-methoxyestradiol inhibits hypoxia-inducible factor 1alpha, tumor growth, and angiogenesis and augments paclitaxel efficacy in head and neck squamous cell carcinoma.Clin Cancer Res. 2004 Dec 15;10(24):8665-73. doi: 10.1158/1078-0432.CCR-04-1393. Clin Cancer Res. 2004. PMID: 15623651
-
Roles of hypoxia, stem cells and epithelial-mesenchymal transition in the spread and treatment resistance of head and neck cancer.J Oral Pathol Med. 2016 Feb;45(2):77-82. doi: 10.1111/jop.12327. Epub 2015 May 7. J Oral Pathol Med. 2016. PMID: 25952002 Review.
Cited by
-
The tumor ecosystem in head and neck squamous cell carcinoma and advances in ecotherapy.Mol Cancer. 2023 Apr 6;22(1):68. doi: 10.1186/s12943-023-01769-z. Mol Cancer. 2023. PMID: 37024932 Free PMC article. Review.
-
Shared Gene Expression and Immune Pathway Changes Associated with Progression from Nevi to Melanoma.Cancers (Basel). 2021 Dec 21;14(1):3. doi: 10.3390/cancers14010003. Cancers (Basel). 2021. PMID: 35008167 Free PMC article.
-
Overexpressed GATA3 enhances the sensitivity of colorectal cancer cells to oxaliplatin through regulating MiR-29b.Cancer Cell Int. 2020 Jul 24;20:339. doi: 10.1186/s12935-020-01424-3. eCollection 2020. Cancer Cell Int. 2020. PMID: 32760217 Free PMC article.
-
Preliminary Interpretations of Epigenetic Profiling of Cord Blood in Preeclampsia.Genes (Basel). 2022 May 16;13(5):888. doi: 10.3390/genes13050888. Genes (Basel). 2022. PMID: 35627272 Free PMC article.
-
Hypoxia-inducible factor-1α: A critical target for inhibiting the metastasis of hepatocellular carcinoma.Oncol Lett. 2022 Jun 28;24(2):284. doi: 10.3892/ol.2022.13404. eCollection 2022 Aug. Oncol Lett. 2022. PMID: 35814827 Free PMC article. Review.
References
-
- Simon MC. Gotta have GATA. Nat Genet 1995; 11: 9–11. - PubMed
-
- Ho IC, Pai SY. GATA-3 – not just for Th2 cells anymore. Cell Mol Immunol 2007; 4: 15–29. - PubMed
-
- Asselin-Labat ML, Sutherland KD, Barker H, Thomas R, Shackleton M, Forrest NC et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol 2007; 9: 201–209. - PubMed
-
- Labastie MC, Catala M, Gregoire JM, Peault B. The GATA-3 gene is expressed during human kidney embryogenesis. Kidney Int 1995; 47: 1597–1603. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

