Simvastatin and atorvastatin reduce the mechanical properties of tendon constructs in vitro and introduce catabolic changes in the gene expression pattern
- PMID: 28264197
- PMCID: PMC5339395
- DOI: 10.1371/journal.pone.0172797
Simvastatin and atorvastatin reduce the mechanical properties of tendon constructs in vitro and introduce catabolic changes in the gene expression pattern
Abstract
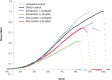
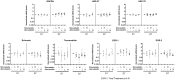
Treatment with lipid-lowering drugs, statins, is common all over the world. Lately, the occurrence of spontaneous tendon ruptures or tendinosis have suggested a negative influence of statins upon tendon tissue. But how statins might influence tendons is not clear. In the present study, we investigated the effect of statin treatment on mechanical strength, cell proliferation, collagen content and gene expression pattern in a tendon-like tissue made from human tenocytes in vitro. Human tendon fibroblasts were grown in a 3D tissue culture model (tendon constructs), and treated with either simvastatin or atorvastatin, low or high dose, respectively, for up to seven days. After seven days of treatment, mechanical testing of the constructs was performed. Collagen content and cell proliferation were also determined. mRNA levels of several target genes were measured after one or seven days. The maximum force and stiffness were reduced by both statins after 7 days (p<0.05), while the cross sectional area was unaffected. Further, the collagen content was reduced by atorvastatin (p = 0.01) and the cell proliferation rate was decreased by both types of statins (p<0.05). Statin treatment also introduced increased mRNA levels of MMP-1, MMP-3, MMP-13, TIMP-1 and decreased levels of collagen type 1 and 3. In conclusion, statin treatment appears to have a negative effect on tendon matrix quality as seen by a reduced strength of the tendon constructs. Further, activated catabolic changes in the gene expression pattern and a reduced collagen content indicated a disturbed balance in matrix production of tendon due to statin administration.
Conflict of interest statement
Figures




Similar articles
-
Effects of statins on matrix metalloproteinases and their endogenous inhibitors in human endothelial cells.Naunyn Schmiedebergs Arch Pharmacol. 2011 Jun;383(6):547-54. doi: 10.1007/s00210-011-0623-0. Epub 2011 Mar 30. Naunyn Schmiedebergs Arch Pharmacol. 2011. PMID: 21448567
-
Do different tendons exhibit the same response following chronic exposure to statins?Can J Physiol Pharmacol. 2017 Apr;95(4):333-339. doi: 10.1139/cjpp-2016-0133. Epub 2016 Oct 4. Can J Physiol Pharmacol. 2017. PMID: 28112540
-
Structural and biomechanical changes in the Achilles tendon after chronic treatment with statins.Food Chem Toxicol. 2015 Mar;77:50-7. doi: 10.1016/j.fct.2014.12.014. Epub 2014 Dec 25. Food Chem Toxicol. 2015. PMID: 25544391
-
MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix.J Appl Physiol (1985). 2013 Sep;115(6):884-91. doi: 10.1152/japplphysiol.00137.2013. Epub 2013 May 2. J Appl Physiol (1985). 2013. PMID: 23640595 Free PMC article. Review.
-
Influence of Statins on Circulating Inflammatory Cytokines in Patients With Abnormal Glucose Homeostasis: A Meta-analysis of Data From Randomized Controlled Trials.Clin Ther. 2020 Feb;42(2):e13-e31. doi: 10.1016/j.clinthera.2019.12.009. Epub 2020 Jan 17. Clin Ther. 2020. PMID: 31955966
Cited by
-
The Plasma Membrane and Mechanoregulation in Cells.ACS Omega. 2024 May 13;9(20):21780-21797. doi: 10.1021/acsomega.4c01962. eCollection 2024 May 21. ACS Omega. 2024. PMID: 38799362 Free PMC article. Review.
-
Shoulder Tendinopathy Induced by Statins: A Case Report and Systematic Review.J Pers Med. 2025 May 15;15(5):198. doi: 10.3390/jpm15050198. J Pers Med. 2025. PMID: 40423069 Free PMC article.
-
Exploring the In Vivo Anti-Inflammatory Actions of Simvastatin-Loaded Porous Microspheres on Inflamed Tenocytes in a Collagenase-Induced Animal Model of Achilles Tendinitis.Int J Mol Sci. 2018 Mar 12;19(3):820. doi: 10.3390/ijms19030820. Int J Mol Sci. 2018. PMID: 29534523 Free PMC article.
-
Statin treatment increases the clinical risk of tendinopathy through matrix metalloproteinase release - a cohort study design combined with an experimental study.Sci Rep. 2019 Nov 29;9(1):17958. doi: 10.1038/s41598-019-53238-7. Sci Rep. 2019. PMID: 31784541 Free PMC article.
-
Investigating the controversy surrounding statin therapy and Achilles tendinopathy using Mendelian randomization analysis.Int J Clin Pharm. 2025 Oct;47(5):1186-1194. doi: 10.1007/s11096-025-01893-4. Epub 2025 Mar 7. Int J Clin Pharm. 2025. PMID: 40053301
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

