Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro
- PMID: 28264445
- PMCID: PMC5372870
- DOI: 10.3390/nu9030207
Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro
Abstract
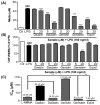
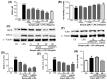
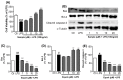
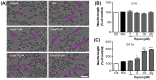
Estrogen deficiency has been well characterized in inflammatory disorders including neuroinflammation. Daidzein, a dietary alternative phytoestrogen found in soy (Glycine max) as primary isoflavones, possess anti-inflammatory activity, but the effect of its active metabolite Equol (7-hydroxy-3-(4'-hydroxyphenyl)-chroman) has not been well established. In this study, we investigated the anti-neuroinflammatory and neuroprotective effect of Equol in vitro. To evaluate the potential effects of Equol, three major types of central nervous system (CNS) cells, including microglia (BV-2), astrocytes (C6), and neurons (N2a), were used. Effects of Equol on the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase (COX-2), Mitogen activated protein kinase (MAPK) signaling proteins, and apoptosis-related proteins were measured by western blot analysis. Equol inhibited the lipopolysaccharide (LPS)-induced TLR4 activation, MAPK activation, NF-kB-mediated transcription of inflammatory mediators, production of nitric oxide (NO), release of prostaglandin E2 (PGE-2), secretion of tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6), in Lipopolysaccharide (LPS)-activated murine microglia cells. Additionally, Equol protects neurons from neuroinflammatory injury mediated by LPS-activated microglia through downregulation of neuronal apoptosis, increased neurite outgrowth in N2a cell and neurotrophins like nerve growth factor (NGF) production through astrocytes further supporting its neuroprotective potential. These findings provide novel insight into the anti-neuroinflammatory effects of Equol on microglial cells, which may have clinical significance in cases of neurodegeneration.
Keywords: Equol; apoptosis; neuroinflammation; neuroprotection; phytoestrogen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

