Silver Nanoparticle-Based Fluorescence-Quenching Lateral Flow Immunoassay for Sensitive Detection of Ochratoxin A in Grape Juice and Wine
- PMID: 28264472
- PMCID: PMC5371838
- DOI: 10.3390/toxins9030083
Silver Nanoparticle-Based Fluorescence-Quenching Lateral Flow Immunoassay for Sensitive Detection of Ochratoxin A in Grape Juice and Wine
Abstract
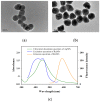
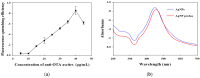
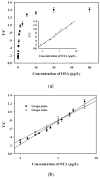
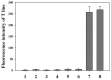
A silver nanoparticle (AgNP)-based fluorescence-quenching lateral flow immunoassay with competitive format (cLFIA) was developed for sensitive detection of ochratoxin A (OTA) in grape juice and wine samples in the present study. The Ru(phen) 3 2 + -doped silica nanoparticles (RuNPs) were sprayed on the test and control line zones as background fluorescence signals. The AgNPs were designed as the fluorescence quenchers of RuNPs because they can block the exciting light transferring to the RuNP molecules. The proposed method exhibited high sensitivity for OTA detection, with a detection limit of 0.06 µg/L under optimized conditions. The method also exhibited a good linear range for OTA quantitative analysis from 0.08 µg/L to 5.0 µg/L. The reliability of the fluorescence-quenching cLFIA method was evaluated through analysis of the OTA-spiked red grape wine and juice samples. The average recoveries ranged from 88.0% to 110.0% in red grape wine and from 92.0% to 110.0% in grape juice. Meanwhile, less than a 10% coefficient variation indicated an acceptable precision of the cLFIA method. In summary, the new AgNP-based fluorescence-quenching cLFIA is a simple, rapid, sensitive, and accurate method for quantitative detection of OTA in grape juice and wine or other foodstuffs.
Keywords: fluorescence quenching; lateral flow immunoassay; ochratoxin A; quantitative detection; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lai W., Fung D.Y.C., Yang X., Renrong L., Xiong Y. Development of a colloidal gold strip for rapid detection of ochratoxin A with mimotope peptide. Food Control. 2009;20:791–795. doi: 10.1016/j.foodcont.2008.10.007. - DOI
-
- Li J., Duan H., Xu P., Huang X., Xiong Y. Effect of different-sized spherical gold nanoparticles grown layer by layer on the sensitivity of an immunochromatographic assay. RSC Adv. 2016;6:26178–26185. doi: 10.1039/C6RA03695C. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

