Retinoic Acid Negatively Impacts Proliferation and MCTC Specific Attributes of Human Skin Derived Mast Cells, but Reinforces Allergic Stimulability
- PMID: 28264498
- PMCID: PMC5372541
- DOI: 10.3390/ijms18030525
Retinoic Acid Negatively Impacts Proliferation and MCTC Specific Attributes of Human Skin Derived Mast Cells, but Reinforces Allergic Stimulability
Abstract
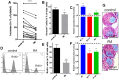
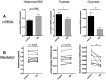
The Vitamin-A-metabolite retinoic acid (RA) acts as a master regulator of cellular programs. Mast cells (MCs) are primary effector cells of type-I-allergic reactions. We recently uncovered that human cutaneous MCs are enriched with RA network components over other skin cells. Yet, direct experimental evidence on the significance of the RA-MC axis is limited. Here, skin-derived cultured MCs were exposed to RA for seven days and investigated by flow-cytometry (BrdU incorporation, Annexin/PI, FcεRI), microscopy, RT-qPCR, histamine quantitation, protease activity, and degranulation assays. We found that while MC size and granularity remained unchanged, RA potently interfered with MC proliferation. Conversely, a modest survival-promoting effect from RA was noted. The granule constituents, histamine and tryptase, remained unaffected, while RA had a striking impact on MC chymase, whose expression dropped by gene and by peptidase activity. The newly uncovered MRGPRX2 performed similarly to chymase. Intriguingly, RA fostered allergic MC degranulation, in a way completely uncoupled from FcεRI expression, but it simultaneously restricted MRGPRX2-triggered histamine release in agreement with the reduced receptor expression. Vitamin-A-derived hormones thus re-shape skin-derived MCs numerically, phenotypically, and functionally. A general theme emerges, implying RA to skew MCs towards processes associated with (allergic) inflammation, while driving them away from the skin-imprinted MCTC ("MCs containing tryptase and chymase") signature (chymase, MRGPRX2). Collectively, MCs are substantial targets of the skin retinoid network.
Keywords: IgER; cell cycle; chymase; mast cell; proliferation; retinoic acid; skin; tryptase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
IL-4 and human skin mast cells revisited: reinforcement of a pro-allergic phenotype upon prolonged exposure.Arch Dermatol Res. 2016 Nov;308(9):665-670. doi: 10.1007/s00403-016-1688-x. Epub 2016 Sep 20. Arch Dermatol Res. 2016. PMID: 27650274
-
Characterization of mast cell subtypes, distribution, and antigen-induced activation in the guinea pig esophagus.Dis Esophagus. 2009;22(7):600-5. doi: 10.1111/j.1442-2050.2009.00944.x. Epub 2009 Feb 13. Dis Esophagus. 2009. PMID: 19222531
-
MRGPRX2 is negatively targeted by SCF and IL-4 to diminish pseudo-allergic stimulation of skin mast cells in culture.Exp Dermatol. 2018 Nov;27(11):1298-1303. doi: 10.1111/exd.13762. Epub 2018 Sep 3. Exp Dermatol. 2018. PMID: 30091263
-
[Elucidation of Mast Cell Activation Mechanism Mediated by Purinergic Signaling].Yakugaku Zasshi. 2021;141(9):1057-1061. doi: 10.1248/yakushi.21-00113. Yakugaku Zasshi. 2021. PMID: 34471006 Review. Japanese.
-
The Role of Mast Cells in IgE-Independent Lung Diseases.Clin Rev Allergy Immunol. 2020 Jun;58(3):377-387. doi: 10.1007/s12016-020-08779-5. Clin Rev Allergy Immunol. 2020. PMID: 32086776 Free PMC article. Review.
Cited by
-
CREB Is Activated by the SCF/KIT Axis in a Partially ERK-Dependent Manner and Orchestrates Survival and the Induction of Immediate Early Genes in Human Skin Mast Cells.Int J Mol Sci. 2023 Feb 18;24(4):4135. doi: 10.3390/ijms24044135. Int J Mol Sci. 2023. PMID: 36835547 Free PMC article.
-
MRGPRX2 Is the Codeine Receptor of Human Skin Mast Cells: Desensitization through β-Arrestin and Lack of Correlation with the FcεRI Pathway.J Invest Dermatol. 2021 May;141(5):1286-1296.e4. doi: 10.1016/j.jid.2020.09.017. Epub 2020 Oct 13. J Invest Dermatol. 2021. PMID: 33058860 Free PMC article.
-
Clorfl86/RHEX Is a Negative Regulator of SCF/KIT Signaling in Human Skin Mast Cells.Cells. 2023 May 3;12(9):1306. doi: 10.3390/cells12091306. Cells. 2023. PMID: 37174705 Free PMC article.
-
β-arrestin-1 and β-arrestin-2 Restrain MRGPRX2-Triggered Degranulation and ERK1/2 Activation in Human Skin Mast Cells.Front Allergy. 2022 Jul 15;3:930233. doi: 10.3389/falgy.2022.930233. eCollection 2022. Front Allergy. 2022. PMID: 35910860 Free PMC article.
-
Thymic Stromal Lymphopoietin Promotes MRGPRX2-Triggered Degranulation of Skin Mast Cells in a STAT5-Dependent Manner with Further Support from JNK.Cells. 2021 Jan 8;10(1):102. doi: 10.3390/cells10010102. Cells. 2021. PMID: 33429916 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

