Screening in Maternity to Ascertain Tuberculosis Status (SMATS) study
- PMID: 28264655
- PMCID: PMC5340038
- DOI: 10.1186/s12879-017-2285-0
Screening in Maternity to Ascertain Tuberculosis Status (SMATS) study
Abstract
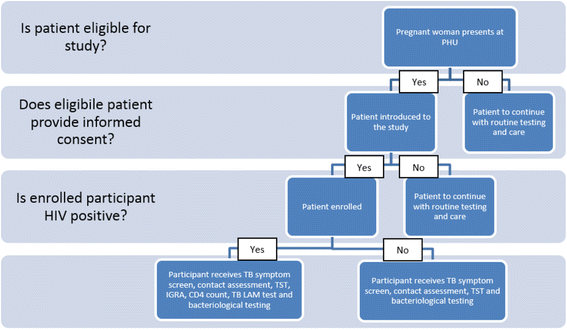
Background: Diagnosis of tuberculosis is difficult among pregnant women because the signs and symptoms of the disease, such as fatigue, shortness of breath, sweating, cough, and mild fever are similar to some manifestations of pregnancy. It is particularly challenging among HIV-infected women as symptoms are often masked or atypical. Currently, WHO recommends a standard four-symptom screening tool for pregnant and lactating women. There is evidence from South Africa that this screening tool (which, despite complex symptomology in this population, recommends identification of patients with weight loss, fever, current cough and night sweats), may be missing true active TB cases. However there exist several laboratory and clinical procedures that have the potential to improve the sensitivity and specificity of this screening tool.
Methods: This study will evaluate the sensitivity and specificity of the current TB screening tool for pregnant and lactating women, both HIV positive and negative. We will also assess several different enhanced screening algorithm using LAM, IGRA, TST and chest radiography and clinical/laboratory procedures and tests. The study will use a cross-sectional analytical study design involving pregnant and lactating women up to six months post-delivery attending antenatal or postnatal care, respectively in one of three selected public health units in Swaziland. Participants will be consecutively enrolled and will be in one of four groups of interest: HIV infected pregnant women, non-HIV infected pregnant women, HIV infected lactating women and non-HIV infected lactating women.
Discussion: We expect in conducting all procedures on all participants regardless of result of the symptom screening we may experience a high refusal rate. However, this risk will be mitigated by the long data collection period of five or more months.
Keywords: Diagnosis; HIV; Pregnancy; Screening; Testing; Tuberculosis.
Figures
References
-
- Factsheet . Tuberculosis in women. Geneva: WHO; 2014.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical