A three-component cognitive behavioural lifestyle program for preconceptional weight-loss in women with polycystic ovary syndrome (PCOS): a protocol for a randomized controlled trial
- PMID: 28264692
- PMCID: PMC5339998
- DOI: 10.1186/s12978-017-0295-4
A three-component cognitive behavioural lifestyle program for preconceptional weight-loss in women with polycystic ovary syndrome (PCOS): a protocol for a randomized controlled trial
Abstract
Background: Obesity in women with polycystic ovary syndrome (PCOS) negatively affects all clinical features, and a 5 to 10% weight loss has shown promising results on reproductive, metabolic and psychological level. Incorporating a healthy diet, increasing physical activity and changing dysfunctional thought patterns in women with PCOS are key points in losing weight. The biggest challenge in weight management programs is to achieve a reasonable and sustainable weight loss. The aim of this study is to explore whether Cognitive Behavioural Therapy (CBT) by a mental health professional, working in a multidisciplinary team with a dietician and a physical therapist (a three-component intervention), is more effective for weight loss in the long term, within 12 months. We will also explore whether mobile phone applications are effective in supporting behavioural change and sustainable weight loss.
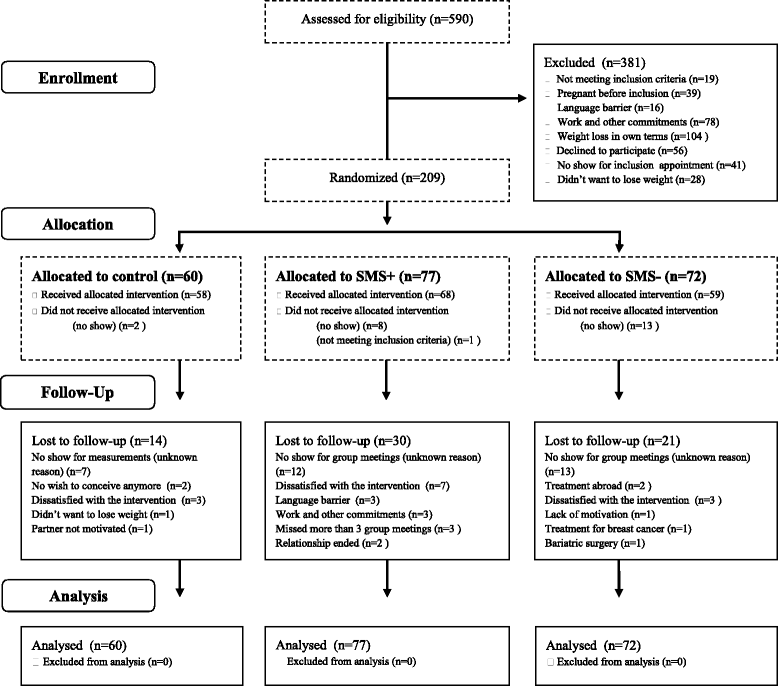
Methods: The present study is a longitudinal randomized controlled trial (RCT) to study the effectiveness of a three-component 1-year cognitive-behavioural lifestyle intervention in overweight/obese women with PCOS. A total of 210 participants are randomly assigned to three groups: 1) CBT provided by the multidisciplinary team or; 2) CBT provided by the multidisciplinary team and Short Message Service (SMS) or; 3) usual care: encourage weight loss through publicly available services (control group). The primary aim of the 12-month intervention is to explore whether a three-component 1-year cognitive-behavioural lifestyle intervention is effective to decrease weight, when compared to usual care. Secondary outcomes include: the effect of the intervention on the PCOS phenotype, waist circumference, waist to hip ratio, ovulation rates, total testosterone, SHBG, free androgen index (FAI), AMH, hirsutism, acne, fasting glucose, blood pressure and all psychological parameters. Additionally, we assessed time to pregnancy, ongoing pregnancies, clinical pregnancies, miscarriages and birth weight. All outcome variables are measured at the start of the study, and again at 3 months, 6 months, nine months and 12 months.
Discussion: We expect that CBT provided by a multidisciplinary team, especially combined with SMS, is effective in developing a healthy lifestyle and achieving a long-term weight loss in women with PCOS. Losing 5- 10% body weight improves various PCOS characteristics. Consequently, we expect to show that CBT provided by a multidisciplinary team improves reproductive and metabolic outcomes, as well as quality of life, while at the same time being cost-effective.
Trial registration: Registered at the Netherlands National Trial Register with number NTR2450 on August 2nd, 2010.
Keywords: CBT; Cognitive therapy; E-health; Life style; Obesity; PCOS; Polycystic ovary syndrome; Quality of life; Text messaging; Weight loss.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous