Critical role of vascular peroxidase 1 in regulating endothelial nitric oxide synthase
- PMID: 28264790
- PMCID: PMC5338721
- DOI: 10.1016/j.redox.2017.02.022
Critical role of vascular peroxidase 1 in regulating endothelial nitric oxide synthase
Abstract
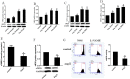
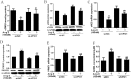
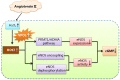
Vascular peroxidase 1 (VPO1) is a member of the peroxidase family which aggravates oxidative stress by producing hypochlorous acid (HOCl). Our previous study demonstrated that VPO1 plays a critical role in endothelial dysfunction through dimethylarginine dimethylaminohydrolase2 (DDAH2)/asymmetric Dimethylarginine (ADMA) pathway. Hereby we describe the regulatory role of VPO1 on endothelial nitric oxide synthase (eNOS) expression and activity in human umbilical vein endothelial cells (HUVECs). In HUVECs AngiotensinII (100nM) treatment reduced Nitric Oxide (NO) production, decreased eNOS expression and activity, which were reversed by VPO1 siRNA. Knockdown of VPO1 also attenuated ADMA production and eNOS uncoupling while enhancing phosphorylated ser1177 eNOS expression level. Furthermore, HOCl stimulation was shown to directly induce ADMA production and eNOS uncoupling, decrease phosphorylated ser1177 eNOS expression. It also significantly suppressed eNOS expression and activity together with NO production. Therefore, VPO1 plays a vital role in regulating eNOS expression and activity via hydrogen peroxide (H2O2)-VPO1-HOCl pathway.
Keywords: Angiotensin II; Asymmetricdimethylarginine; Endothelial nitric oxide synthase; Nitric oxide; Oxidative stress; Vascular peroxidase 1.
Copyright © 2017. Published by Elsevier B.V.
Figures


References
-
- Peng H., Chen L., Huang X. Vascular peroxidase 1 up regulation by angiotensin II attenuates nitric oxide production through increasing asymmetrical dimethylarginine in HUVECs. J. Am. Soc. Hypertens. 2016;10(9):741–751. - PubMed
-
- Onozato M.L., Tojo A., Leiper J. Expression of NG,NG-dimethylarginine dimethylaminohydrolase and protein arginine N-methyltransferase isoforms in diabetic rat kidney: effects of angiotensin II receptor blockers. Diabetes. 2008;57(1):172–180. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

