Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohort study
- PMID: 28264814
- PMCID: PMC6168035
- DOI: 10.1136/bmj.j895
Risk of serious infections associated with use of immunosuppressive agents in pregnant women with autoimmune inflammatory conditions: cohort study
Abstract
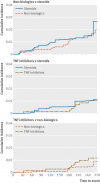
Objective To compare the risk of serious infections associated with use of systemic steroids, non-biologic agents, or tumor necrosis factor α (TNF) inhibitors in pregnancy.Design Observational cohort study.Setting Public (Medicaid, 2001-10) or private (Optum Clinformatics, 2004-15) health insurance programs in the US.Participants 4961 pregnant women treated with immunosuppressive drugs for rheumatoid arthritis, systemic lupus erythematosus, ankylosing spondylitis, psoriatic arthritis, or inflammatory bowel disease.Exposure for observational studies Exposure was classified into steroid, non-biologic, or TNF inhibitors on first filled prescription during pregnancy. Because TNF inhibitors are not used to treat systemic lupus erythematosus, patients with this condition were excluded from comparisons involving TNF inhibitors.Main outcome measure The main outcome was occurrence of serious infections during pregnancy, defined by hospital admission for bacterial or opportunistic infections. Hazard ratios were derived using Cox proportional hazard regression models after adjustment for confounding with propensity score fine stratification. A logistic regression model was used to conduct a dose-response analysis among women filling at least one steroid prescription.Results 71 out of 4961 pregnant women (0.2%) treated with immunosuppressive agents experienced serious infections. The crude incidence rates of serious infections per 100 person years among 2598 steroid users, 1587 non-biologic users, and 776 TNF inhibitors users included in this study were 3.4 (95% confidence interval 2.5 to 4.7), 2.3 (1.5 to 3.5), and 1.5 (0.7 to 3.0), respectively. No statistically significant differences in the risk of serious infections during pregnancy were observed among users of the three immunosuppressive drug classes: non-biologics v steroids, hazard ratio 0.81 (95% confidence interval 0.48 to 1.37), TNF inhibitors v steroids 0.91 (0.36 to 2.26), and TNF inhibitors v non-biologics 1.36 (0.47 to 3.93). In the dose-response analysis, higher steroid dose was associated with an increased risk of serious infections during pregnancy (coefficient for each unit increase in average prednisone equivalent mg daily dose=0.019, P=0.02).Conclusions Risk of serious infections is similar among pregnant women with systemic inflammatory conditions using steroids, non-biologics, and TNF inhibitors. However, high dose steroid use is an independent risk factor of serious infections in pregnancy.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
