Role of Polarity Proteins in the Generation and Organization of Apical Surface Protrusions
- PMID: 28264821
- PMCID: PMC5587363
- DOI: 10.1101/cshperspect.a027813
Role of Polarity Proteins in the Generation and Organization of Apical Surface Protrusions
Abstract
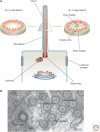
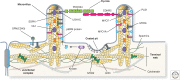
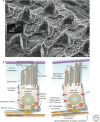
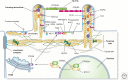
Protruding from the apical surfaces of epithelial cells are specialized structures, including cilia, microplicae, microvilli, and stereocilia. These contribute to epithelial function by cushioning the apical surface, by amplifying its surface area to facilitate nutrient absorption, and by promoting sensory transduction and barrier function. Despite these important roles, and the diseases that result when their formation is perturbed, there remain significant gaps in our understanding of the biogenesis of apical protrusions, or the pathways that promote their organization and orientation once at the apical surface. Here, I review some general aspects of these apical structures, and then discuss our current understanding of their formation and organization with respect to proteins that specify apicobasolateral polarity and planar cell polarity.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures




Similar articles
-
Rewiring cell polarity signaling in cancer.Oncogene. 2015 Feb 19;34(8):939-50. doi: 10.1038/onc.2014.59. Epub 2014 Mar 17. Oncogene. 2015. PMID: 24632617 Review.
-
Ultramicroscopic examination of the ovine tonsillar epithelia.Anat Rec (Hoboken). 2010 May;293(5):879-89. doi: 10.1002/ar.21098. Anat Rec (Hoboken). 2010. PMID: 20225209
-
Structure, regulation, and functional diversity of microvilli on the apical domain of epithelial cells.Annu Rev Cell Dev Biol. 2015;31:593-621. doi: 10.1146/annurev-cellbio-100814-125234. Annu Rev Cell Dev Biol. 2015. PMID: 26566117 Review.
-
The keratin-binding protein Albatross regulates polarization of epithelial cells.J Cell Biol. 2008 Oct 6;183(1):19-28. doi: 10.1083/jcb.200803133. J Cell Biol. 2008. PMID: 18838552 Free PMC article.
-
Emerging role for epithelial polarity proteins of the Crumbs family as potential tumor suppressors.J Biomed Biotechnol. 2011;2011:868217. doi: 10.1155/2011/868217. Epub 2011 Sep 6. J Biomed Biotechnol. 2011. PMID: 21912482 Free PMC article. Review.
Cited by
-
Identification of a novel RPGR mutation associated with retinitis pigmentosa and primary ciliary dyskinesia in a Slovak family: a case report.Front Pediatr. 2024 Jan 25;12:1339664. doi: 10.3389/fped.2024.1339664. eCollection 2024. Front Pediatr. 2024. PMID: 38333087 Free PMC article.
-
The Urothelium: Life in a Liquid Environment.Physiol Rev. 2020 Oct 1;100(4):1621-1705. doi: 10.1152/physrev.00041.2019. Epub 2020 Mar 19. Physiol Rev. 2020. PMID: 32191559 Free PMC article. Review.
-
Hydroxylated sphingolipid biosynthesis regulates photoreceptor apical domain morphogenesis.J Cell Biol. 2020 Dec 7;219(12):e201911100. doi: 10.1083/jcb.201911100. J Cell Biol. 2020. PMID: 33048164 Free PMC article.
-
Development and Clinical Validation of a Seven-Gene Prognostic Signature Based on Multiple Machine Learning Algorithms in Kidney Cancer.Cell Transplant. 2021 Jan-Dec;30:963689720969176. doi: 10.1177/0963689720969176. Cell Transplant. 2021. PMID: 33626918 Free PMC article.
-
A deep learning framework for quantitative analysis of actin microridges.NPJ Syst Biol Appl. 2023 Jun 2;9(1):21. doi: 10.1038/s41540-023-00276-7. NPJ Syst Biol Appl. 2023. PMID: 37268613 Free PMC article.
References
-
- Adler PN, Zhu C, Stone D. 2004. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Curr Biol 14: 2046–2051. - PubMed
-
- Al Jord A, Lemaitre AI, Delgehyr N, Faucourt M, Spassky N, Meunier A. 2014. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature 516: 104–107. - PubMed
-
- Ameen N, Apodaca G. 2007. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic 8: 998–1006. - PubMed
-
- Anderson RA. 1977. Actin filaments in normal and migrating corneal epithelial cells. Invest Ophthalmol Vis Sci 16: 161–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
