Computer-determined dosage of insulin in the management of neonatal hyperglycaemia (HINT2): protocol of a randomised controlled trial
- PMID: 28264826
- PMCID: PMC5353287
- DOI: 10.1136/bmjopen-2016-012982
Computer-determined dosage of insulin in the management of neonatal hyperglycaemia (HINT2): protocol of a randomised controlled trial
Abstract
Introduction: Neonatal hyperglycaemia is frequently treated with insulin, which may increase the risk of hypoglycaemia. Computer-determined dosage of insulin (CDD) with the STAR-GRYPHON program uses a computer model to predict an effective dose of insulin to treat hyperglycaemia while minimising the risk of hypoglycaemia. However, CDD models can require more frequent blood glucose testing than common clinical protocols. The aim of this trial is to determine if CDD using STAR-GRYPHON reduces hypoglycaemia in hyperglycaemic preterm babies treated with insulin independent of the frequency of blood glucose testing.
Methods and analysis: Design: Multicentre, non-blinded, randomised controlled trial.
Setting: Neonatal intensive care units in New Zealand and Australia.
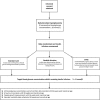
Participants: 138 preterm babies ≤30 weeks' gestation or ≤1500 g at birth who develop hyperglycaemia (two consecutive blood glucose concentrations ≥10 mmol/L, at least 4 hours apart) will be randomised to one of three groups: (1) CDD using the STAR-GRYPHON model-based decision support system: insulin dose and frequency of blood glucose testing advised by STAR-GRYPHON, with a maximum testing interval of 4 hours; (2) bedside titration: insulin dose determined by medical staff, maximum blood glucose testing interval of 4 hours; (3) standard care: insulin dose and frequency of blood glucose testing determined by medical staff. The target range for blood glucose concentrations is 5-8 mmol/L in all groups. A subset of babies will have masked continuous glucose monitoring.
Primary outcome: is the number of babies with one or more episodes of hypoglycaemia (blood glucose concentration <2.6 mmol/L), during treatment with insulin.
Ethics and dissemination: This protocol has been approved by New Zealand's Health and Disability Ethics Committee: 14/STH/26. A data safety monitoring committee has been appointed to oversee the trial. Findings will be disseminated to participants and carers, peer-reviewed journals, guideline developers and the public.
Trial registration number: 12614000492651.
Keywords: Biological model; Blood glucose; Clinical decision support system; Hypoglycaemia; Preterm Infant.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Similar articles
-
Safe and effective glycaemic control in premature infants: observational clinical results from the computerised STAR-GRYPHON protocol.Arch Dis Child Fetal Neonatal Ed. 2019 Mar;104(2):F205-F211. doi: 10.1136/archdischild-2017-314072. Epub 2018 Jun 21. Arch Dis Child Fetal Neonatal Ed. 2019. PMID: 29930148
-
Real-time continuous glucose monitoring in preterm infants (REACT): an international, open-label, randomised controlled trial.Lancet Child Adolesc Health. 2021 Apr;5(4):265-273. doi: 10.1016/S2352-4642(20)30367-9. Epub 2021 Feb 10. Lancet Child Adolesc Health. 2021. PMID: 33577770 Free PMC article. Clinical Trial.
-
Protocol of a randomised controlled trial of real-time continuous glucose monitoring in neonatal intensive care 'REACT'.BMJ Open. 2018 Jun 4;8(6):e020816. doi: 10.1136/bmjopen-2017-020816. BMJ Open. 2018. PMID: 29866729 Free PMC article.
-
Continuous glucose monitoring in extremely preterm infants in intensive care: the REACT RCT and pilot study of ‘closed-loop’ technology.Southampton (UK): NIHR Journals Library; 2021 Oct. Southampton (UK): NIHR Journals Library; 2021 Oct. PMID: 34723449 Free Books & Documents. Review.
-
Management of hyperglycaemia in the preterm infant.Arch Dis Child Fetal Neonatal Ed. 2010 Mar;95(2):F126-31. doi: 10.1136/adc.2008.154716. Arch Dis Child Fetal Neonatal Ed. 2010. PMID: 20231218 Review.
Cited by
-
Continuous glucose monitoring in the neonatal intensive care unit: not quite ready for 'plug and play'.Arch Dis Child Fetal Neonatal Ed. 2019 Jul;104(4):F344-F345. doi: 10.1136/archdischild-2018-315899. Epub 2018 Nov 13. Arch Dis Child Fetal Neonatal Ed. 2019. PMID: 30425111 Free PMC article. No abstract available.
-
Improving glycemic control in critically ill patients: personalized care to mimic the endocrine pancreas.Crit Care. 2018 Aug 2;22(1):182. doi: 10.1186/s13054-018-2110-1. Crit Care. 2018. PMID: 30071851 Free PMC article. Review.
References
-
- New Zealand Health Information Service. Maternity snapshot 2010: Ministry of Health; 2011. http://www.health.govt.nz/publication/maternity-snapshot-2010-provisiona... (accessed 28 Feb 2017)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical