IFT56 regulates vertebrate developmental patterning by maintaining IFTB complex integrity and ciliary microtubule architecture
- PMID: 28264835
- PMCID: PMC5399663
- DOI: 10.1242/dev.143255
IFT56 regulates vertebrate developmental patterning by maintaining IFTB complex integrity and ciliary microtubule architecture
Abstract
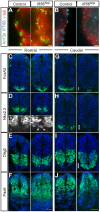
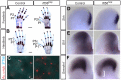
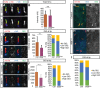
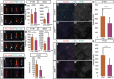
Cilia are key regulators of animal development and depend on intraflagellar transport (IFT) proteins for their formation and function, yet the roles of individual IFT proteins remain unclear. We examined the Ift56hop mouse mutant and reveal novel insight into the function of IFT56, a poorly understood IFTB protein. Ift56hop mice have normal cilia distribution but display defective cilia structure, including abnormal positioning and number of ciliary microtubule doublets. We show that Ift56hop cilia are unable to accumulate Gli proteins efficiently, resulting in developmental patterning defects in Shh signaling-dependent tissues such as the limb and neural tube. Strikingly, core IFTB proteins are unable to accumulate normally within Ift56hop cilia, including IFT88, IFT81 and IFT27, which are crucial for key processes such as tubulin transport and Shh signaling. IFT56 is required specifically for the IFTB complex, as IFTA components and proteins that rely on IFTA function are unaffected in Ift56hop cilia. These studies define a distinct and novel role for IFT56 in IFTB complex integrity that is crucial for cilia structure and function and, ultimately, animal development.
Keywords: Cilia; Hedgehog signaling; IFT56; IFTB; Intraflagellar transport; Microtubule structure; TTC26.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
Similar articles
-
A mutation in the mouse ttc26 gene leads to impaired hedgehog signaling.PLoS Genet. 2014 Oct 23;10(10):e1004689. doi: 10.1371/journal.pgen.1004689. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25340710 Free PMC article.
-
The graded response to Sonic Hedgehog depends on cilia architecture.Dev Cell. 2007 May;12(5):767-78. doi: 10.1016/j.devcel.2007.03.004. Dev Cell. 2007. PMID: 17488627
-
THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia.Nat Genet. 2008 Apr;40(4):403-410. doi: 10.1038/ng.105. Epub 2008 Mar 9. Nat Genet. 2008. PMID: 18327258 Free PMC article.
-
Gli proteins in development and disease.Annu Rev Cell Dev Biol. 2011;27:513-37. doi: 10.1146/annurev-cellbio-092910-154048. Epub 2011 Jul 21. Annu Rev Cell Dev Biol. 2011. PMID: 21801010 Review.
-
Cilia and developmental signaling.Annu Rev Cell Dev Biol. 2007;23:345-73. doi: 10.1146/annurev.cellbio.23.090506.123249. Annu Rev Cell Dev Biol. 2007. PMID: 17506691 Free PMC article. Review.
Cited by
-
Biliary, Renal, Neurological, and Skeletal syndrome in a Chinese boy.Pediatr Nephrol. 2025 Apr;40(4):947-949. doi: 10.1007/s00467-024-06591-3. Epub 2024 Nov 8. Pediatr Nephrol. 2025. PMID: 39514123
-
The ciliary protein RPGRIP1L governs autophagy independently of its proteasome-regulating function at the ciliary base in mouse embryonic fibroblasts.Autophagy. 2018;14(4):567-583. doi: 10.1080/15548627.2018.1429874. Epub 2018 Feb 21. Autophagy. 2018. PMID: 29372668 Free PMC article.
-
Study on gene expression in stomach at different developmental stages of human embryos.Front Cell Dev Biol. 2025 May 30;13:1564789. doi: 10.3389/fcell.2025.1564789. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40519260 Free PMC article.
-
Intraflagellar Transport Proteins as Regulators of Primary Cilia Length.Front Cell Dev Biol. 2021 May 19;9:661350. doi: 10.3389/fcell.2021.661350. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095126 Free PMC article. Review.
-
Mutations in Ciliary Trafficking Genes affect Sonic Hedgehog-dependent Neural Tube Patterning Differentially along the Anterior-Posterior Axis.Neuroscience. 2020 Dec 1;450:3-14. doi: 10.1016/j.neuroscience.2020.07.015. Epub 2020 Jul 16. Neuroscience. 2020. PMID: 32682825 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

