High Prevalence and Predominance of the aph(2″)-If Gene Conferring Aminoglycoside Resistance in Campylobacter
- PMID: 28264854
- PMCID: PMC5404564
- DOI: 10.1128/AAC.00112-17
High Prevalence and Predominance of the aph(2″)-If Gene Conferring Aminoglycoside Resistance in Campylobacter
Abstract
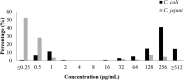
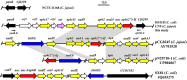
Campylobacter is a major foodborne pathogen, and previous studies revealed that Campylobacter isolates from food-producing animals are increasingly resistant to gentamicin in China. The molecular epidemiology and genetic mechanisms responsible for gentamicin resistance in China have not been well understood. In this study, 607 Campylobacter isolates of chicken and swine origins collected in 2014 were analyzed, revealing that 15.6% (25/160) of the Campylobacter jejuni isolates and 79.9% (357/447) of the Campylobacter coli isolates were resistant to gentamicin. PCR detection of the gentamicin resistance genes indicated that aph(2″)-If was more prevalent than the previously identified aacA/aphD gene and has become the dominant gentamicin resistance determinant in Campylobacter Transformation and whole-genome sequencing as well as long-range PCR discovered that aph(2″)-If was located on a chromosomal segment inserted between two conserved genes, Cj0299 and panB Cloning of aph(2″)-If into gentamicin-susceptible C. jejuni NCTC 11168 confirmed its function in conferring high-level resistance to gentamicin and kanamycin. Molecular typing by pulsed-field gel electrophoresis suggested that both regional expansion of a particular clone and horizontal transmission were involved in the dissemination of the aph(2″)-If gene in Campylobacter To our knowledge, this is the first report describing the high prevalence of a chromosomally encoded aph(2″)-If gene in Campylobacter The high prevalence and predominance of this gene might be driven by the use of aminoglycoside antibiotics in food animal production in China and potentially compromise the usefulness of gentamicin as a therapeutic agent for Campylobacter-associated systemic infection.
Keywords: Campylobacter; aph(2″)-If; food safety; gentamicin resistance.
Copyright © 2017 American Society for Microbiology.
Figures

Similar articles
-
Novel gentamicin resistance genes in Campylobacter isolated from humans and retail meats in the USA.J Antimicrob Chemother. 2015 May;70(5):1314-21. doi: 10.1093/jac/dkv001. Epub 2015 Feb 1. J Antimicrob Chemother. 2015. PMID: 25645207 Free PMC article.
-
Transmission of dominant strains of Campylobacter jejuni and Campylobacter coli between farms and retail stores in Ecuador: Genetic diversity and antimicrobial resistance.PLoS One. 2024 Sep 24;19(9):e0308030. doi: 10.1371/journal.pone.0308030. eCollection 2024. PLoS One. 2024. PMID: 39316598 Free PMC article.
-
Whole-genome sequencing of gentamicin-resistant Campylobacter coli isolated from U.S. retail meats reveals novel plasmid-mediated aminoglycoside resistance genes.Antimicrob Agents Chemother. 2013 Nov;57(11):5398-405. doi: 10.1128/AAC.00669-13. Epub 2013 Aug 19. Antimicrob Agents Chemother. 2013. PMID: 23959310 Free PMC article.
-
The Current State of Macrolide Resistance in Campylobacter spp.: Trends and Impacts of Resistance Mechanisms.Appl Environ Microbiol. 2017 May 31;83(12):e00416-17. doi: 10.1128/AEM.00416-17. Print 2017 Jun 15. Appl Environ Microbiol. 2017. PMID: 28411226 Free PMC article. Review.
-
Campylobacter coli Prosthetic Joint Infection: Case Report and a Review of the Literature.Pathogens. 2024 Sep 27;13(10):838. doi: 10.3390/pathogens13100838. Pathogens. 2024. PMID: 39452710 Free PMC article. Review.
Cited by
-
Aminoglycoside Resistance and Possible Mechanisms in Campylobacter Spp. Isolated From Chicken and Swine in Jiangsu, China.Front Microbiol. 2021 Oct 8;12:716185. doi: 10.3389/fmicb.2021.716185. eCollection 2021. Front Microbiol. 2021. PMID: 34690960 Free PMC article.
-
Differences in the Propensity of Different Antimicrobial Resistance Determinants to Be Disseminated via Transformation in Campylobacter jejuni and Campylobacter coli.Microorganisms. 2022 Jun 10;10(6):1194. doi: 10.3390/microorganisms10061194. Microorganisms. 2022. PMID: 35744712 Free PMC article.
-
Emerging erm(B)-Mediated Macrolide Resistance Associated with Novel Multidrug Resistance Genomic Islands in Campylobacter.Antimicrob Agents Chemother. 2019 Jun 24;63(7):e00153-19. doi: 10.1128/AAC.00153-19. Print 2019 Jul. Antimicrob Agents Chemother. 2019. PMID: 31085517 Free PMC article.
-
Emergence of Erythromycin Resistance Methyltransferases in Campylobacter coli Strains in France.Antimicrob Agents Chemother. 2021 Oct 18;65(11):e0112421. doi: 10.1128/AAC.01124-21. Epub 2021 Aug 9. Antimicrob Agents Chemother. 2021. PMID: 34370579 Free PMC article.
-
Prediction of antimicrobial resistance in clinical Campylobacter jejuni isolates from whole-genome sequencing data.Eur J Clin Microbiol Infect Dis. 2021 Apr;40(4):673-682. doi: 10.1007/s10096-020-04043-y. Epub 2020 Sep 24. Eur J Clin Microbiol Infect Dis. 2021. PMID: 32974772 Free PMC article.
References
-
- Blaser MJ, Engberg J. 2008. Clinical aspects of Campylobacter jejuni and Campylobacter coli infections, p 99–121. In Nachamkin I, Szymanski CM, Blaser MJ (ed), Campylobacter, 3rd ed ASM Press, Washington, DC.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

