Differences in the Importance of Mast Cells, Basophils, IgE, and IgG versus That of CD4+ T Cells and ILC2 Cells in Primary and Secondary Immunity to Strongyloides venezuelensis
- PMID: 28264908
- PMCID: PMC5400847
- DOI: 10.1128/IAI.00053-17
Differences in the Importance of Mast Cells, Basophils, IgE, and IgG versus That of CD4+ T Cells and ILC2 Cells in Primary and Secondary Immunity to Strongyloides venezuelensis
Abstract
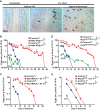
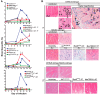
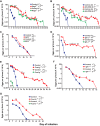
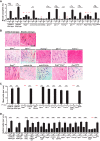
There is evidence that mast cells, basophils, and IgE can contribute to immune responses to parasites; however, the relative levels of importance of these effector elements in parasite immunity are not fully understood. Previous work in Il3-deficient and c-kit mutant KitW/W-v mice indicated that interleukin-3 and c-Kit contribute to expulsion of the intestinal nematode Strongyloides venezuelensis during primary infection. Our findings in mast cell-deficient KitW-sh/W-sh mice and two types of mast cell-deficient mice that have normal c-kit ("Hello Kitty" and MasTRECK mice) confirmed prior work in KitW/W-v mice that suggested that mast cells play an important role in S. venezuelensis egg clearance in primary infections. We also assessed a possible contribution of basophils in immune responses to S. venezuelensis By immunohistochemistry, we found that numbers of basophils and mast cells were markedly increased in the jejunal mucosa during primary infections with S. venezuelensis Studies in basophil-deficient Mcpt8DTR mice revealed a small but significant contribution of basophils to S. venezuelensis egg clearance in primary infections. Studies in mice deficient in various components of immune responses showed that CD4+ T cells and ILC2 cells, IgG, FcRγ, and, to a lesser extent, IgE and FcεRI contribute to effective immunity in primary S. venezuelensis infections. These findings support the conclusion that the hierarchy of importance of immune effector mechanisms in primary S. venezuelensis infection is as follows: CD4+ T cells/ILC2 cells, IgG, and FcRγ>mast cells>IgE and FcεRI>basophils. In contrast, in secondary S. venezuelensis infection, our evidence indicates that the presence of CD4+ T cells is of critical importance but mast cells, antibodies, and basophils have few or no nonredundant roles.
Keywords: Immunoglobulin E; basophils; mast cells; parasites.
Copyright © 2017 American Society for Microbiology.
Figures


Similar articles
-
IgG and IgE collaboratively accelerate expulsion of Strongyloides venezuelensis in a primary infection.Infect Immun. 2013 Jul;81(7):2518-27. doi: 10.1128/IAI.00285-13. Epub 2013 Apr 29. Infect Immun. 2013. PMID: 23630966 Free PMC article.
-
Basophils are dispensable for the establishment of protective adaptive immunity against primary and challenge infection with the intestinal helminth parasite Strongyloides ratti.PLoS Negl Trop Dis. 2018 Nov 29;12(11):e0006992. doi: 10.1371/journal.pntd.0006992. eCollection 2018 Nov. PLoS Negl Trop Dis. 2018. PMID: 30496188 Free PMC article.
-
Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites.Nature. 1998 Mar 5;392(6671):90-3. doi: 10.1038/32190. Nature. 1998. PMID: 9510253
-
Mast cells and basophils.Curr Opin Hematol. 2000 Jan;7(1):32-9. doi: 10.1097/00062752-200001000-00007. Curr Opin Hematol. 2000. PMID: 10608502 Review.
-
Roles of basophils and mast cells in cutaneous inflammation.Semin Immunopathol. 2016 Sep;38(5):563-70. doi: 10.1007/s00281-016-0570-4. Epub 2016 May 11. Semin Immunopathol. 2016. PMID: 27170045 Review.
Cited by
-
Skin Mast Cells Contribute to Sporothrix schenckii Infection.Front Immunol. 2020 Mar 19;11:469. doi: 10.3389/fimmu.2020.00469. eCollection 2020. Front Immunol. 2020. PMID: 32265923 Free PMC article.
-
Systemic Immune Modulation by Gastrointestinal Nematodes.Annu Rev Immunol. 2024 Jun;42(1):259-288. doi: 10.1146/annurev-immunol-090222-101331. Epub 2024 Jun 14. Annu Rev Immunol. 2024. PMID: 38277692 Free PMC article. Review.
-
Communication is key: Innate immune cells regulate host protection to helminths.Front Immunol. 2022 Sep 26;13:995432. doi: 10.3389/fimmu.2022.995432. eCollection 2022. Front Immunol. 2022. PMID: 36225918 Free PMC article. Review.
-
Mast Cells: Fascinating but Still Elusive after 140 Years from Their Discovery.Int J Mol Sci. 2020 Jan 11;21(2):464. doi: 10.3390/ijms21020464. Int J Mol Sci. 2020. PMID: 31940755 Free PMC article.
-
ILC2s in infectious diseases and organ-specific fibrosis.Semin Immunopathol. 2018 Jul;40(4):379-392. doi: 10.1007/s00281-018-0677-x. Epub 2018 Mar 26. Semin Immunopathol. 2018. PMID: 29623414 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

