Missing-in-metastasis protein downregulates CXCR4 by promoting ubiquitylation and interaction with small Rab GTPases
- PMID: 28264927
- PMCID: PMC5399787
- DOI: 10.1242/jcs.198937
Missing-in-metastasis protein downregulates CXCR4 by promoting ubiquitylation and interaction with small Rab GTPases
Abstract
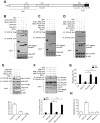
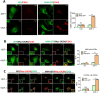
Surface expression of chemokine receptor CXCR4 is downregulated by missing-in-metastasis protein (MIM; also known as MTSS1), a member of the inverse BAR (I-BAR)-domain protein family that recognizes and generates membranes with negative curvature. Yet, the mechanism for the regulation is unknown. Here, we show that MIM forms a complex with CXCR4 by binding to E3 ubiquitin ligase AIP4 (also known as ITCH) in response to stromal cell-derived factor 1 (SDF-1; also known as CXCL12). Overexpression of MIM promoted CXCR4 ubiquitylation, inhibited cellular response to SDF-1, caused accumulation and aggregation of multivesicular bodies (MVBs) in the cytoplasm, and promoted CXCR4 sorting into MVBs in a manner depending on binding to AIP4. In response to SDF-1, MIM also bound transiently to the small GTPase Rab5 at 5 min and to Rab7 at 30 min. Binding to Rab7 requires an N-terminal coiled-coil motif, deletion of which abolished MIM-mediated MVB formation and CXCR4 internalization. Our results unveil a previously unknown property of MIM that establishes the linkage of protein ubiquitylation with Rab-guided trafficking of CXCR4 in endocytic vesicles.
Keywords: AIP4; CXCR4; MIM; MVBs; Rab7; Ubiquitylation.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

