Inhibition of coactivator-associated arginine methyltransferase 1 modulates dendritic arborization and spine maturation of cultured hippocampal neurons
- PMID: 28264928
- PMCID: PMC5391767
- DOI: 10.1074/jbc.M117.775619
Inhibition of coactivator-associated arginine methyltransferase 1 modulates dendritic arborization and spine maturation of cultured hippocampal neurons
Abstract
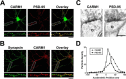
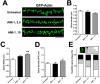
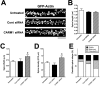
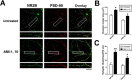
An improved understanding of the molecular mechanisms in synapse formation provides insight into both learning and memory and the etiology of neurodegenerative disorders. Coactivator-associated arginine methyltransferase 1 (CARM1) is a protein methyltransferase that negatively regulates synaptic gene expression and inhibits neuronal differentiation. Despite its regulatory function in neurons, little is known about the CARM1 cellular location and its role in dendritic maturation and synapse formation. Here, we examined the effects of CARM1 inhibition on dendritic spine and synapse morphology in the rat hippocampus. CARM1 was localized in hippocampal post-synapses, with immunocytochemistry and electron microscopy revealing co-localization of CARM1 with post-synaptic density (PSD)-95 protein, a post-synaptic marker. Specific siRNA-mediated suppression of CARM1 expression resulted in precocious dendritic maturation, with increased spine width and density at sites along dendrites and induction of mushroom-type spines. These changes were accompanied by a striking increase in the cluster size and number of key synaptic proteins, including N-methyl-d-aspartate receptor subunit 2B (NR2B) and PSD-95. Similarly, pharmacological inhibition of CARM1 activity with the CARM1-specific inhibitor AMI-1 significantly increased spine width and mushroom-type spines and also increased the cluster size and number of NR2B and cluster size of PSD-95. These results suggest that CARM1 is a post-synaptic protein that plays roles in dendritic maturation and synaptic formation and that spatiotemporal regulation of CARM1 activity modulates neuronal connectivity and improves synaptic dysfunction.
Keywords: CARM1; PSD; dendritic maturation; dendritic spine; hippocampal neurons; hippocampus; neuron; signal transduction; synapse; synaptic clustering.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
PSD95 suppresses dendritic arbor development in mature hippocampal neurons by occluding the clustering of NR2B-NMDA receptors.PLoS One. 2014 Apr 4;9(4):e94037. doi: 10.1371/journal.pone.0094037. eCollection 2014. PLoS One. 2014. PMID: 24705401 Free PMC article.
-
Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines.J Biol Chem. 2013 Mar 1;288(9):6438-50. doi: 10.1074/jbc.M112.412478. Epub 2013 Jan 8. J Biol Chem. 2013. PMID: 23300088 Free PMC article.
-
Postsynaptic density 95 (PSD-95) serine 561 phosphorylation regulates a conformational switch and bidirectional dendritic spine structural plasticity.J Biol Chem. 2017 Sep 29;292(39):16150-16160. doi: 10.1074/jbc.M117.782490. Epub 2017 Aug 8. J Biol Chem. 2017. PMID: 28790172 Free PMC article.
-
Making of a Synapse: Recurrent Roles of Drebrin A at Excitatory Synapses Throughout Life.Adv Exp Med Biol. 2017;1006:119-139. doi: 10.1007/978-4-431-56550-5_8. Adv Exp Med Biol. 2017. PMID: 28865018 Review.
-
The thorny side of addiction: adaptive plasticity and dendritic spines.ScientificWorldJournal. 2007 Nov 2;7:9-21. doi: 10.1100/tsw.2007.247. ScientificWorldJournal. 2007. PMID: 17982573 Free PMC article. Review.
Cited by
-
Gallic Acid Impedes Non-Small Cell Lung Cancer Progression via Suppression of EGFR-Dependent CARM1-PELP1 Complex.Drug Des Devel Ther. 2020 Apr 23;14:1583-1592. doi: 10.2147/DDDT.S228123. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32425504 Free PMC article.
-
The Role of Protein Arginine Methylation as Post-Translational Modification on Actin Cytoskeletal Components in Neuronal Structure and Function.Cells. 2021 May 1;10(5):1079. doi: 10.3390/cells10051079. Cells. 2021. PMID: 34062765 Free PMC article. Review.
-
Recent advances in branching mechanisms underlying neuronal morphogenesis.F1000Res. 2018 Nov 12;7:F1000 Faculty Rev-1779. doi: 10.12688/f1000research.16038.1. eCollection 2018. F1000Res. 2018. PMID: 30473771 Free PMC article. Review.
-
Small Cell Lung Cancer Neuronal Features and Their Implications for Tumor Progression, Metastasis, and Therapy.Mol Cancer Res. 2024 Sep 4;22(9):787-795. doi: 10.1158/1541-7786.MCR-24-0265. Mol Cancer Res. 2024. PMID: 38912893 Free PMC article. Review.
-
Arginine Methylation in Brain Tumors: Tumor Biology and Therapeutic Strategies.Cells. 2021 Jan 11;10(1):124. doi: 10.3390/cells10010124. Cells. 2021. PMID: 33440687 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

