Emerging role of dopamine in neovascularization of pheochromocytoma and paraganglioma
- PMID: 28264974
- PMCID: PMC5434646
- DOI: 10.1096/fj.201601131R
Emerging role of dopamine in neovascularization of pheochromocytoma and paraganglioma
Abstract
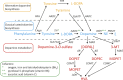
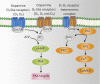
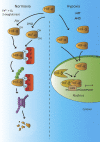
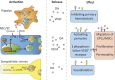
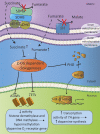
Dopamine is a catecholamine that acts both as a neurotransmitter and as a hormone, exerting its functions via dopamine (DA) receptors that are present in a broad variety of organs and cells throughout the body. In the circulation, DA is primarily stored in and transported by blood platelets. Recently, the important contribution of DA in the regulation of angiogenesis has been recognized. In vitro and in vivo studies have shown that DA inhibits angiogenesis through activation of the DA receptor type 2. Overproduction of catecholamines is the biochemical hallmark of pheochromocytoma (PCC) and paraganglioma (PGL). The increased production of DA has been shown to be an independent predictor of malignancy in these tumors. The precise relationship underlying the association between DA production and PCC and PGL behavior needs further clarification. Herein, we review the biochemical and physiologic aspects of DA with a focus on its relations with VEGF and hypoxia inducible factor related angiogenesis pathways, with special emphasis on DA producing PCC and PGL.-Osinga, T. E., Links, T. P., Dullaart, R. P. F., Pacak, K., van der Horst-Schrivers, A. N. A., Kerstens, M. N., Kema, I. P. Emerging role of dopamine in neovascularization of pheochromocytoma and paraganglioma.
Keywords: VEGF; angiogenesis; hypoxia-inducible factor.
© FASEB.
Figures

Similar articles
-
Hypoxia Pathway Mutations in Pheochromocytomas and Paragangliomas.Cytogenet Genome Res. 2016;150(3-4):227-241. doi: 10.1159/000457479. Epub 2017 Feb 24. Cytogenet Genome Res. 2016. PMID: 28231563 Review.
-
Surgical outcome of laparoscopic surgery, including laparoendoscopic single-site surgery, for retroperitoneal paraganglioma compared with adrenal pheochromocytoma.J Endourol. 2014 Jun;28(6):686-92. doi: 10.1089/end.2013.0706. Epub 2014 Mar 24. J Endourol. 2014. PMID: 24499341
-
Somatic mutations in H-RAS in sporadic pheochromocytoma and paraganglioma identified by exome sequencing.J Clin Endocrinol Metab. 2013 Jul;98(7):E1266-71. doi: 10.1210/jc.2012-4257. Epub 2013 May 2. J Clin Endocrinol Metab. 2013. PMID: 23640968
-
Pheochromocytoma/Paraganglioma: A Poster Child for Cancer Metabolism.J Clin Endocrinol Metab. 2018 May 1;103(5):1779-1789. doi: 10.1210/jc.2017-01991. J Clin Endocrinol Metab. 2018. PMID: 29409060 Review.
-
Phenylethanolamine N-methyltransferase downregulation is associated with malignant pheochromocytoma/paraganglioma.Oncotarget. 2016 Apr 26;7(17):24141-53. doi: 10.18632/oncotarget.8234. Oncotarget. 2016. PMID: 27007161 Free PMC article.
Cited by
-
The dopaminergic control of Cushing's syndrome.J Endocrinol Invest. 2022 Jul;45(7):1297-1315. doi: 10.1007/s40618-021-01661-x. Epub 2022 Apr 23. J Endocrinol Invest. 2022. PMID: 35460460 Free PMC article. Review.
-
A Novel Dop2/Invertebrate-Type Dopamine Signaling System Potentially Mediates Stress, Female Reproduction, and Early Development in the Pacific Oyster (Crassostrea gigas).Mar Biotechnol (NY). 2021 Oct;23(5):683-694. doi: 10.1007/s10126-021-10052-5. Epub 2021 Aug 7. Mar Biotechnol (NY). 2021. PMID: 34365528
-
[Advances in pharmacological research for retinopathy of prematurity].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025 May 25;54(3):411-421. doi: 10.3724/zdxbyxb-2024-0216. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2025. PMID: 40394926 Free PMC article. Review. Chinese.
-
Dopamine Alleviated Diabetic Retinal Neurodegeneration through Protecting Retinal Ganglion Cells from Ferroptosis via the Nrf2/HO‑1 Signaling Pathway.ACS Omega. 2025 Jul 14;10(28):30397-30410. doi: 10.1021/acsomega.5c02063. eCollection 2025 Jul 22. ACS Omega. 2025. PMID: 40727724 Free PMC article.
-
Overexpression of dopamine receptor D2 promotes colorectal cancer progression by activating the β-catenin/ZEB1 axis.Cancer Sci. 2021 Sep;112(9):3732-3743. doi: 10.1111/cas.15026. Epub 2021 Jul 4. Cancer Sci. 2021. Retraction in: Cancer Sci. 2023 Jul;114(7):3056. doi: 10.1111/cas.15838. PMID: 34118099 Free PMC article. Retracted.
References
-
- Lenders J. W. M., Eisenhofer G., Mannelli M., Pacak K. (2005) Phaeochromocytoma. Lancet 366, 665–675 - PubMed
-
- Mcmillan M. (1956) Identification of hydroxytyramine in a chromaffin tumour. Lancet 268, 284 - PubMed
-
- Eisenhofer G., Lenders J. W. M., Siegert G., Bornstein S. R., Friberg P., Milosevic D., Mannelli M., Linehan W. M., Adams K., Timmers H. J. L. M., Pacak K. (2012) Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur. J. Cancer 48, 1739–1749 - PMC - PubMed
-
- Eisenhofer G., Tischler A. S., de Krijger R. R. (2012) Diagnostic tests and biomarkers for pheochromocytoma and extra-adrenal paraganglioma: from routine laboratory methods to disease stratification. Endocr. Pathol. 23, 4–14 - PubMed
-
- Blank A., Schmitt A. M., Korpershoek E., van Nederveen F., Rudolph T., Weber N., Strebel R. T., de Krijger R., Komminoth P., Perren A. (2010) SDHB loss predicts malignancy in pheochromocytomas/sympathethic paragangliomas, but not through hypoxia signalling. Endocr. Relat. Cancer 17, 919–928 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

