Constant Light Desynchronizes Olfactory versus Object and Visuospatial Recognition Memory Performance
- PMID: 28264977
- PMCID: PMC5373134
- DOI: 10.1523/JNEUROSCI.3213-16.2017
Constant Light Desynchronizes Olfactory versus Object and Visuospatial Recognition Memory Performance
Abstract
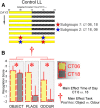
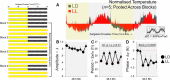
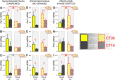
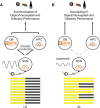
Circadian rhythms optimize physiology and behavior to the varying demands of the 24 h day. The master circadian clock is located in the suprachiasmatic nuclei (SCN) of the hypothalamus and it regulates circadian oscillators in tissues throughout the body to prevent internal desynchrony. Here, we demonstrate for the first time that, under standard 12 h:12 h light/dark (LD) cycles, object, visuospatial, and olfactory recognition performance in C57BL/6J mice is consistently better at midday relative to midnight. However, under repeated exposure to constant light (rLL), recognition performance becomes desynchronized, with object and visuospatial performance better at subjective midday and olfactory performance better at subjective midnight. This desynchrony in behavioral performance is mirrored by changes in expression of the canonical clock genes Period1 and Period2 (Per1 and Per2), as well as the immediate-early gene Fos in the SCN, dorsal hippocampus, and olfactory bulb. Under rLL, rhythmic Per1 and Fos expression is attenuated in the SCN. In contrast, hippocampal gene expression remains rhythmic, mirroring object and visuospatial performance. Strikingly, Per1 and Fos expression in the olfactory bulb is reversed, mirroring the inverted olfactory performance. Temporal desynchrony among these regions does not result in arrhythmicity because core body temperature and exploratory activity rhythms persist under rLL. Our data provide the first demonstration that abnormal lighting conditions can give rise to temporal desynchrony between autonomous circadian oscillators in different regions, with different consequences for performance across different sensory domains. Such a dispersed network of dissociable circadian oscillators may provide greater flexibility when faced with conflicting environmental signals.SIGNIFICANCE STATEMENT A master circadian clock in the suprachiasmatic nuclei (SCN) of the hypothalamus regulates physiology and behavior across the 24 h day by synchronizing peripheral clocks throughout the brain and body. Without the SCN, these peripheral clocks rapidly become desynchronized. Here, we provide a unique demonstration that, under lighting conditions in which the central clock in the SCN is dampened, peripheral oscillators in the hippocampus and olfactory bulb become desynchronized, along with the behavioral processes mediated by these clocks. Multiple clocks that adopt different phase relationships may enable processes occurring in different brain regions to be optimized to specific phases of the 24 h day. Moreover, such a dispersed network of dissociable circadian clocks may provide greater flexibility when faced with conflicting environmental signals (e.g., seasonal changes in photoperiod).
Keywords: circadian; clock genes; hippocampus; internal desynchrony; olfactory bulb; suprachiasmatic nuclei.
Copyright © 2017 Tam et al.
Figures

Similar articles
-
Disrupted light-dark cycle abolishes circadian expression of peripheral clock genes without inducing behavioral arrhythmicity in mice.Biochem Biophys Res Commun. 2015 Mar 6;458(2):256-61. doi: 10.1016/j.bbrc.2015.01.095. Epub 2015 Jan 31. Biochem Biophys Res Commun. 2015. PMID: 25645021
-
Advanced light-entrained activity onsets and restored free-running suprachiasmatic nucleus circadian rhythms in per2/dec mutant mice.Chronobiol Int. 2011 Nov;28(9):737-50. doi: 10.3109/07420528.2011.607374. Chronobiol Int. 2011. PMID: 22080784
-
Circadian PER2::LUC rhythms in the olfactory bulb of freely moving mice depend on the suprachiasmatic nucleus but not on behaviour rhythms.Eur J Neurosci. 2015 Dec;42(12):3128-37. doi: 10.1111/ejn.13111. Eur J Neurosci. 2015. PMID: 26489367
-
Regulation and function of extra-SCN circadian oscillators in the brain.Acta Physiol (Oxf). 2020 May;229(1):e13446. doi: 10.1111/apha.13446. Epub 2020 Feb 6. Acta Physiol (Oxf). 2020. PMID: 31965726 Review.
-
Peripheral circadian oscillators in mammals.Handb Exp Pharmacol. 2013;(217):45-66. doi: 10.1007/978-3-642-25950-0_3. Handb Exp Pharmacol. 2013. PMID: 23604475 Review.
Cited by
-
Dim light in the evening causes coordinated realignment of circadian rhythms, sleep, and short-term memory.Proc Natl Acad Sci U S A. 2021 Sep 28;118(39):e2101591118. doi: 10.1073/pnas.2101591118. Proc Natl Acad Sci U S A. 2021. PMID: 34556572 Free PMC article.
-
The medial prefrontal cortex - hippocampus circuit that integrates information of object, place and time to construct episodic memory in rodents: Behavioral, anatomical and neurochemical properties.Neurosci Biobehav Rev. 2020 Jun;113:373-407. doi: 10.1016/j.neubiorev.2020.04.007. Epub 2020 Apr 13. Neurosci Biobehav Rev. 2020. PMID: 32298711 Free PMC article. Review.
-
Diurnal rhythm regulates the frequency of carbachol-induced beta oscillation via inhibitory neural system in rat hippocampus.Cogn Neurodyn. 2022 Jun;16(3):507-518. doi: 10.1007/s11571-021-09736-4. Epub 2021 Nov 10. Cogn Neurodyn. 2022. PMID: 35603053 Free PMC article.
-
Sex-specific attenuation of constant light-induced memory impairment and Clock gene expression in brain in hepatic Npas2 knockout mice.Sci Rep. 2025 Mar 11;15(1):8347. doi: 10.1038/s41598-025-92938-1. Sci Rep. 2025. PMID: 40069567 Free PMC article.
-
Light and the laboratory mouse.J Neurosci Methods. 2018 Apr 15;300:26-36. doi: 10.1016/j.jneumeth.2017.04.007. Epub 2017 Apr 14. J Neurosci Methods. 2018. PMID: 28414048 Free PMC article.
References
-
- Ang G. (2016) Investigating sleep and circadian rhythm disruptions in models of schizophrenia. Doctoral thesis, University of Oxford.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
