Dopamine Inhibition Differentially Controls Excitability of Substantia Nigra Dopamine Neuron Subpopulations through T-Type Calcium Channels
- PMID: 28264982
- PMCID: PMC5373143
- DOI: 10.1523/JNEUROSCI.0117-17.2017
Dopamine Inhibition Differentially Controls Excitability of Substantia Nigra Dopamine Neuron Subpopulations through T-Type Calcium Channels
Abstract
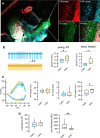
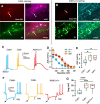
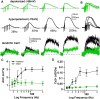
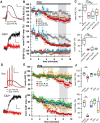
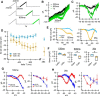
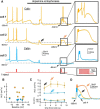
While there is growing appreciation for diversity among ventral tegmental area dopamine neurons, much less is known regarding functional heterogeneity among the substantia nigra pars compacta (SNc) neurons. Here, we show that calbindin-positive dorsal tier and calbindin-negative ventral tier SNc dopaminergic neurons in mice comprise functionally distinct subpopulations distinguished by their dendritic calcium signaling, rebound excitation, and physiological responses to dopamine D2-receptor (D2) autoinhibition. While dopamine is known to inhibit action potential backpropagation, our experiments revealed an unexpected enhancement of excitatory responses and dendritic calcium signals in the presence of D2-receptor inhibition. Specifically, dopamine inhibition and direct hyperpolarization enabled the generation of low-threshold depolarizations that occurred in an all-or-none or graded manner, due to recruitment of T-type calcium channels. Interestingly, these effects occurred selectively in calbindin-negative dopaminergic neurons within the SNc. Thus, calbindin-positive and calbindin-negative SNc neurons differ substantially in their calcium channel composition and efficacy of excitatory inputs in the presence of dopamine inhibition.SIGNIFICANCE STATEMENT Substantia nigra dopaminergic neurons can be divided into two populations: the calbindin-negative ventral tier, which is vulnerable to neurodegeneration in Parkinson's disease, and the calbindin-positive dorsal tier, which is relatively resilient. Although tonic firing is similar in these subpopulations, we find that their responses to dopamine-mediated inhibition are strikingly different. During inhibition, calbindin-negative neurons exhibit increased sensitivity to excitatory inputs, which can then trigger large dendritic calcium transients due to strong expression of T-type calcium channels. Therefore, SNc neurons differ substantially in their calcium channel composition, which may contribute to their differential vulnerability. Furthermore, T-currents increase excitation efficacy onto calbindin-negative cells during dopamine inhibition, suggesting that shared inputs are differentially processed in subpopulations resulting in distinct downstream dopamine signals.
Keywords: calbindin; dendrites; dopamine; substantia nigra; t-type calcium channels; two-photon imaging.
Copyright © 2017 the authors 0270-6474/17/373704-17$15.00/0.
Figures




References
-
- Aizenman CD, Linden DJ (1999) Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol 82:1697–1709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
