Absent Filling of Ipsilateral Superficial Middle Cerebral Vein Is Associated With Poor Outcome After Reperfusion Therapy
- PMID: 28265013
- PMCID: PMC5374052
- DOI: 10.1161/STROKEAHA.116.016174
Absent Filling of Ipsilateral Superficial Middle Cerebral Vein Is Associated With Poor Outcome After Reperfusion Therapy
Abstract
Background and purpose: Our aim was to study the effect of drainage of cortical veins, including the superficial middle cerebral vein (SMCV), vein of Trolard, and vein of Labbé on neurological outcomes after reperfusion therapy.
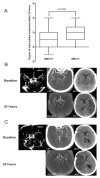
Methods: Consecutive ischemic stroke patients who underwent pretreatment computed tomographic perfusion and 24-hour computed tomographic perfusion or magnetic resonance perfusion after intravenous thrombolysis were included. We defined "absent filling of ipsilateral cortical vein" (eg, SMCV-) as no contrast filling of the vein across the whole venous phase on 4-dimensional computed tomographic angiography in the ischemic hemisphere.
Results: Of 228 patients, SMCV-, vein of Trolard- and vein of Labbé- were observed in 50 (21.9%), 27 (11.8%), and 32 (14.0%) patients, respectively. Only SMCV- independently predicted poor outcome (3-month modified Rankin Scale score of >2; odds ratio, 2.710; P=0.040). No difference was found in reperfusion rate after treatment between patients with and without SMCV- (P>0.05). In patients achieving major reperfusion (≥80%), there was no difference in 24-hour infarct volume, or rate of poor outcome between patients with and without SMCV- (P>0.05). However, in those without major reperfusion, patients with SMCV- had larger 24-hour infarct volume (P=0.011), higher rate of poor outcome (P=0.012), and death (P=0.032) compared with those with SMCV filling. SMCV- was significantly associated with brain edema at 24 hours (P=0.037), which, in turn, was associated with poor 3-month outcome (P=0.002).
Conclusions: Lack of SMCV filling contributed to poor outcome after thrombolysis, especially when reperfusion was not achieved. The main deleterious effect of poor venous filling appears related to the development of brain edema.
Keywords: brain imaging; edema; outcome; reperfusion; vein.
© 2017 American Heart Association, Inc.
Figures



References
-
- Ishikawa M, Kusaka G, Yamaguchi N, Sekizuka E, Nakadate H, Minamitani H, et al. Platelet and leukocyte adhesion in the microvasculature at the cerebral surface immediately after subarachnoid hemorrhage. Neurosurgery. 2009;64:546–553. discussion 553-544. - PubMed
-
- van den Wijngaard IR, Wermer MJ, Boiten J, Algra A, Holswilder G, Meijer FJ, et al. Cortical venous filling on dynamic computed tomographic angiography: A novel predictor of clinical outcome in patients with acute middle cerebral artery stroke. Stroke. 2016;47:762–767. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

