Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons
- PMID: 28265061
- PMCID: PMC5373408
- DOI: 10.1073/pnas.1614462114
Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons
Abstract
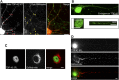
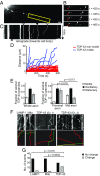
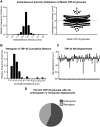
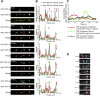
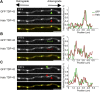
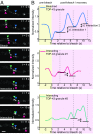
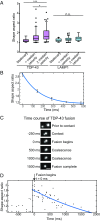
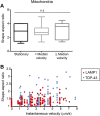
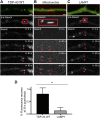
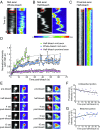
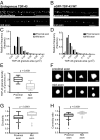
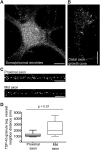
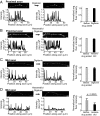
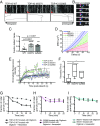
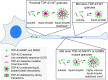
Ribonucleoprotein (RNP) granules are enriched in specific RNAs and RNA-binding proteins (RBPs) and mediate critical cellular processes. Purified RBPs form liquid droplets in vitro through liquid-liquid phase separation and liquid-like non-membrane-bound structures in cells. Mutations in the human RBPs TAR-DNA binding protein 43 (TDP-43) and RNA-binding protein FUS cause amyotrophic lateral sclerosis (ALS), but the biophysical properties of these proteins have not yet been studied in neurons. Here, we show that TDP-43 RNP granules in axons of rodent primary cortical neurons display liquid-like properties, including fusion with rapid relaxation to circular shape, shear stress-induced deformation, and rapid fluorescence recovery after photobleaching. RNP granules formed from wild-type TDP-43 show distinct biophysical properties depending on axonal location, suggesting maturation to a more stabilized structure is dependent on subcellular context, including local density and aging. Superresolution microscopy demonstrates that the stabilized population of TDP-43 RNP granules in the proximal axon is less circular and shows spiculated edges, whereas more distal granules are both more spherical and more dynamic. RNP granules formed by ALS-linked mutant TDP-43 are more viscous and exhibit disrupted transport dynamics. We propose these altered properties may confer toxic gain of function and reflect differential propensity for pathological transformation.
Keywords: TDP-43; amyotrophic lateral sclerosis; liquid droplets; neurons; ribonucleoprotein granules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

