Reconstitution of Saccharomyces cerevisiae DNA polymerase ε-dependent mismatch repair with purified proteins
- PMID: 28265089
- PMCID: PMC5389320
- DOI: 10.1073/pnas.1701753114
Reconstitution of Saccharomyces cerevisiae DNA polymerase ε-dependent mismatch repair with purified proteins
Abstract
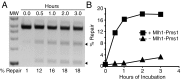
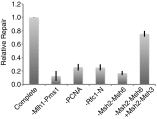
Mammalian and Saccharomyces cerevisiae mismatch repair (MMR) proteins catalyze two MMR reactions in vitro. In one, mispair binding by either the MutS homolog 2 (Msh2)-MutS homolog 6 (Msh6) or the Msh2-MutS homolog 3 (Msh3) stimulates 5' to 3' excision by exonuclease 1 (Exo1) from a single-strand break 5' to the mispair, excising the mispair. In the other, Msh2-Msh6 or Msh2-Msh3 activate the MutL homolog 1 (Mlh1)-postmeiotic segregation 1 (Pms1) endonuclease in the presence of a mispair and a nick 3' to the mispair, to make nicks 5' to the mispair, allowing Exo1 to excise the mispair. DNA polymerase δ (Pol δ) is thought to catalyze DNA synthesis to fill in the gaps resulting from mispair excision. However, colocalization of the S. cerevisiae mispair recognition proteins with the replicative DNA polymerases during DNA replication has suggested that DNA polymerase ε (Pol ε) may also play a role in MMR. Here we describe the reconstitution of Pol ε-dependent MMR using S. cerevisiae proteins. A mixture of Msh2-Msh6 (or Msh2-Msh3), Exo1, RPA, RFC-Δ1N, PCNA, and Pol ε was found to catalyze both short-patch and long-patch 5' nick-directed MMR of a substrate containing a +1 (+T) mispair. When the substrate contained a nick 3' to the mispair, a mixture of Msh2-Msh6 (or Msh2-Msh3), Exo1, RPA, RFC-Δ1N, PCNA, and Pol ε was found to catalyze an MMR reaction that required Mlh1-Pms1. These results demonstrate that Pol ε can act in eukaryotic MMR in vitro.
Keywords: DNA excision; DNA repair; DNA replication fidelity; genome instability; mutator phenotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


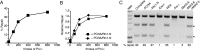
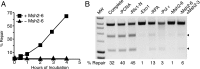
Comment in
-
The Devil is in the details for DNA mismatch repair.Proc Natl Acad Sci U S A. 2017 Apr 4;114(14):3552-3554. doi: 10.1073/pnas.1702747114. Epub 2017 Mar 29. Proc Natl Acad Sci U S A. 2017. PMID: 28356513 Free PMC article. No abstract available.
Similar articles
-
Activation of Saccharomyces cerevisiae Mlh1-Pms1 Endonuclease in a Reconstituted Mismatch Repair System.J Biol Chem. 2015 Aug 28;290(35):21580-90. doi: 10.1074/jbc.M115.662189. Epub 2015 Jul 13. J Biol Chem. 2015. PMID: 26170454 Free PMC article.
-
Mispair-specific recruitment of the Mlh1-Pms1 complex identifies repair substrates of the Saccharomyces cerevisiae Msh2-Msh3 complex.J Biol Chem. 2014 Mar 28;289(13):9352-64. doi: 10.1074/jbc.M114.552190. Epub 2014 Feb 18. J Biol Chem. 2014. PMID: 24550389 Free PMC article.
-
The properties of Msh2-Msh6 ATP binding mutants suggest a signal amplification mechanism in DNA mismatch repair.J Biol Chem. 2018 Nov 23;293(47):18055-18070. doi: 10.1074/jbc.RA118.005439. Epub 2018 Sep 20. J Biol Chem. 2018. PMID: 30237169 Free PMC article.
-
Eukaryotic DNA mismatch repair.Curr Opin Genet Dev. 1999 Feb;9(1):89-96. doi: 10.1016/s0959-437x(99)80013-6. Curr Opin Genet Dev. 1999. PMID: 10072354 Review.
-
DNA mismatch repair and mutation avoidance pathways.J Cell Physiol. 2002 Apr;191(1):28-41. doi: 10.1002/jcp.10077. J Cell Physiol. 2002. PMID: 11920679 Review.
Cited by
-
Genomic Instability Promoted by Overexpression of Mismatch Repair Factors in Yeast: A Model for Understanding Cancer Progression.Genetics. 2018 Jun;209(2):439-456. doi: 10.1534/genetics.118.300923. Epub 2018 Apr 13. Genetics. 2018. PMID: 29654124 Free PMC article.
-
The mismatch repair endonuclease MutLα tethers duplex regions of DNA together and relieves DNA torsional tension.Nucleic Acids Res. 2023 Apr 11;51(6):2725-2739. doi: 10.1093/nar/gkad096. Nucleic Acids Res. 2023. PMID: 36840719 Free PMC article.
-
The Devil is in the details for DNA mismatch repair.Proc Natl Acad Sci U S A. 2017 Apr 4;114(14):3552-3554. doi: 10.1073/pnas.1702747114. Epub 2017 Mar 29. Proc Natl Acad Sci U S A. 2017. PMID: 28356513 Free PMC article. No abstract available.
-
A Human MSH6 Germline Variant Associated With Systemic Lupus Erythematosus Induces Lupus-like Disease in Mice.ACR Open Rheumatol. 2022 Sep;4(9):760-770. doi: 10.1002/acr2.11471. Epub 2022 Jun 16. ACR Open Rheumatol. 2022. PMID: 35708944 Free PMC article.
-
In vivo CRISPR-Cas9 genome editing in mice identifies genetic modifiers of somatic CAG repeat instability in Huntington's disease.Nat Genet. 2025 Feb;57(2):314-322. doi: 10.1038/s41588-024-02054-5. Epub 2025 Jan 22. Nat Genet. 2025. PMID: 39843658 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

