Myosin filament activation in the heart is tuned to the mechanical task
- PMID: 28265101
- PMCID: PMC5373356
- DOI: 10.1073/pnas.1619484114
Myosin filament activation in the heart is tuned to the mechanical task
Abstract
The mammalian heart pumps blood through the vessels, maintaining the dynamic equilibrium in a circulatory system driven by two pumps in series. This vital function is based on the fine-tuning of cardiac performance by the Frank-Starling mechanism that relates the pressure exerted by the contracting ventricle (end systolic pressure) to its volume (end systolic volume). At the level of the sarcomere, the structural unit of the cardiac myocytes, the Frank-Starling mechanism consists of the increase in active force with the increase of sarcomere length (length-dependent activation). We combine sarcomere mechanics and micrometer-nanometer-scale X-ray diffraction from synchrotron light in intact ventricular trabeculae from the rat to measure the axial movement of the myosin motors during the diastole-systole cycle under sarcomere length control. We find that the number of myosin motors leaving the off, ATP hydrolysis-unavailable state characteristic of the diastole is adjusted to the sarcomere length-dependent systolic force. This mechanosensing-based regulation of the thick filament makes the energetic cost of the systole rapidly tuned to the mechanical task, revealing a prime aspect of the Frank-Starling mechanism. The regulation is putatively impaired by cardiomyopathy-causing mutations that affect the intramolecular and intermolecular interactions controlling the off state of the motors.
Keywords: Frank–Starling mechanism; cardiac muscle; heart regulation; myosin filament mechanosensing; small-angle X-ray diffraction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
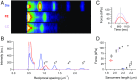

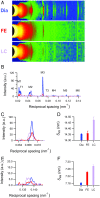
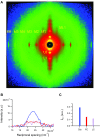
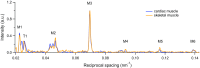
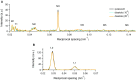

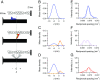
References
-
- Huxley HE, Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967;30(2):383–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

