Remodeling Brain Activity by Repetitive Cervicothoracic Transspinal Stimulation after Human Spinal Cord Injury
- PMID: 28265259
- PMCID: PMC5316528
- DOI: 10.3389/fneur.2017.00050
Remodeling Brain Activity by Repetitive Cervicothoracic Transspinal Stimulation after Human Spinal Cord Injury
Abstract
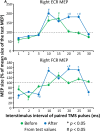
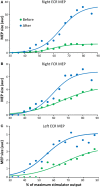
Interventions that can produce targeted brain plasticity after human spinal cord injury (SCI) are needed for restoration of impaired movement in these patients. In this study, we tested the effects of repetitive cervicothoracic transspinal stimulation in one person with cervical motor incomplete SCI on cortical and corticospinal excitability, which were assessed via transcranial magnetic stimulation with paired and single pulses, respectively. We found that repetitive cervicothoracic transspinal stimulation potentiated intracortical facilitation in flexor and extensor wrist muscles, recovered intracortical inhibition in the more impaired wrist flexor muscle, increased corticospinal excitability bilaterally, and improved voluntary muscle strength. These effects may have been mediated by improvements in cortical integration of ascending sensory inputs and strengthening of corticospinal connections. Our novel therapeutic intervention opens new avenues for targeted brain neuromodulation protocols in individuals with cervical motor incomplete SCI.
Keywords: cortical plasticity; corticospinal plasticity; primary motor cortex; repetitive transspinal stimulation; spinal cord injury.
Figures


References
-
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain (1994) 117(4):847–58. - PubMed
-
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology (1997) 48(5):1398–403. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

