Role of Streptococcus pneumoniae Proteins in Evasion of Complement-Mediated Immunity
- PMID: 28265264
- PMCID: PMC5316553
- DOI: 10.3389/fmicb.2017.00224
Role of Streptococcus pneumoniae Proteins in Evasion of Complement-Mediated Immunity
Abstract
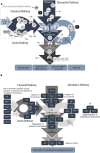
The complement system plays a central role in immune defense against Streptococcus pneumoniae. In order to evade complement attack, pneumococci have evolved a number of mechanisms that limit complement mediated opsonization and subsequent phagocytosis. This review focuses on the strategies employed by pneumococci to circumvent complement mediated immunity, both in vitro and in vivo. At last, since many of the proteins involved in interactions with complement components are vaccine candidates in different stages of validation, we explore the use of these antigens alone or in combination, as potential vaccine approaches that aim at elimination or drastic reduction in the ability of this bacterium to evade complement.
Keywords: Streptococcus pneumoniae; complement system; pneumococcal moonlighting proteins; protein-based vaccines; virulence factors pneumococcal surface proteins.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

