Integrating insulin-like growth factor 1 and sex hormones into neuroprotection: Implications for diabetes
- PMID: 28265342
- PMCID: PMC5320748
- DOI: 10.4239/wjd.v8.i2.45
Integrating insulin-like growth factor 1 and sex hormones into neuroprotection: Implications for diabetes
Abstract
Brain integrity and cognitive aptitude are often impaired in patients with diabetes mellitus, presumably a result of the metabolic complications inherent to the disease. However, an increasing body of evidence has demonstrated the central role of insulin-like growth factor 1 (IGF1) and its relation to sex hormones in many neuroprotective processes. Both male and female patients with diabetes display abnormal IGF1 and sex-hormone levels but the comparison of these fluctuations is seldom a topic of interest. It is interesting to note that both IGF1 and sex hormones have the ability to regulate phosphoinositide 3-kinase-Akt and mitogen-activated protein kinases-extracellular signal-related kinase signaling cascades in animal and cell culture models of neuroprotection. Additionally, there is considerable evidence demonstrating the neuroprotective coupling of IGF1 and estrogen. Androgens have also been implicated in many neuroprotective processes that operate on similar signaling cascades as the estrogen-IGF1 relation. Yet, androgens have not been directly linked to the brain IGF1 system and neuroprotection. Despite the sex-specific variations in brain integrity and hormone levels observed in diabetic patients, the IGF1-sex hormone relation in neuroprotection has yet to be fully substantiated in experimental models of diabetes. Taken together, there is a clear need for the comprehensive analysis of sex differences on brain integrity of diabetic patients and the relationship between IGF1 and sex hormones that may influence brain-health outcomes. As such, this review will briefly outline the basic relation of diabetes and IGF1 and its role in neuroprotection. We will also consider the findings on sex hormones and diabetes as a basis for separately analyzing males and females to identify possible hormone-induced brain abnormalities. Finally, we will introduce the neuroprotective interplay of IGF1 and estrogen and how androgen-derived neuroprotection operates through similar signaling cascades. Future research on both neuroprotection and diabetes should include androgens into the interplay of IGF1 and sex hormones.
Keywords: Androgens; Brain integrity; Cognition; Diabetes; Estrogen; Insulin; Insulin-like growth factor 1; Neuroprotection.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Figures
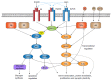
References
-
- Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, et al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD - summary. Diab Vasc Dis Res. 2014;11:133–173. - PubMed
-
- Tillin T, Hughes AD, Mayet J, Whincup P, Sattar N, Forouhi NG, McKeigue PM, Chaturvedi N. The relationship between metabolic risk factors and incident cardiovascular disease in Europeans, South Asians, and African Caribbeans: SABRE (Southall and Brent Revisited) -- a prospective population-based study. J Am Coll Cardiol. 2013;61:1777–1786. - PMC - PubMed
-
- van Elderen SG, de Roos A, de Craen AJ, Westendorp RG, Blauw GJ, Jukema JW, Bollen EL, Middelkoop HA, van Buchem MA, van der Grond J. Progression of brain atrophy and cognitive decline in diabetes mellitus: a 3-year follow-up. Neurology. 2010;75:997–1002. - PubMed
-
- Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

