Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors
- PMID: 28265355
- PMCID: PMC5332256
- DOI: 10.1002/fsn3.383
Euglena gracilis paramylon activates human lymphocytes by upregulating pro-inflammatory factors
Abstract
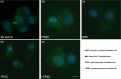
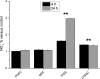
The aim of this study was to verify the activation details and products of human lymphomonocytes, stimulated by different β-glucans, that is Euglena paramylon, MacroGard®, and lipopolysaccharide. We investigated the gene expression of inflammation-related cytokines and mediators, transactivation of relevant transcription factors, and phagocytosis role in cell-glucan interactions, by means of RT-PCR, immunocytochemistry, and colorimetric assay. Our results show that sonicated and alkalized paramylon upregulates pro-inflammatory factors (NO, TNF-α, IL-6, and COX-2) in lymphomonocytes. A clear demonstration of this upregulation is the increased transactivation of NF-kB visualized by immunofluorescence microscopy. Phagocytosis assay showed that internalization is not a mandatory step for signaling cascade to be triggered, since immune activity is not present in the lymphomonocytes that have internalized paramylon granules and particulate MacroGard®. Moreover, the response of Euglena β-glucan-activated lymphomonocytes is much greater than that induced by commercially used β-glucans such as MacroGard®. Our in vitro results indicate that linear fibrous Euglena β-glucan, obtained by sonication and alkaline treatment can act as safe and effective coadjutant of the innate immune system response.
Keywords: Euglena; MacroGard®; glucans; innate immunity; paramylon.
Figures



References
-
- Alaban, L. R. S. , Andrino K. G. S., Ojerio V. T., Seterra E. L., and Corre V. L.. 2014. Effect of β glucan immersion on the survival of mud crab Scylla serrata (Portunidae) larvae. ABAH Bioflux 6:168–172.
-
- Alexander, C. , and Rietschel E. T.. 2001. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 7:167–202. - PubMed
-
- Baert, K. , Sonck E., Goddeeris B. M., Devriendt B., and Cox E.. 2015. Cell type‐specific differences in β‐glucan recognition and signalling in porcine innate immune cells. Dev. Comp. Immunol. 48:192–203. - PubMed
-
- Barsanti, L. , Vismara R., Passarelli V., and Gualtieri P.. 2001. Paramylon (β‐1,3‐glucan) content in wild type and WZSL mutant of Euglena gracilis: effects of growth conditions. J. Appl. Phycol. 13:59–65.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

