Twice-Daily versus Once-Daily Pramipexole Extended Release Dosage Regimens in Parkinson's Disease
- PMID: 28265478
- PMCID: PMC5318624
- DOI: 10.1155/2017/8518929
Twice-Daily versus Once-Daily Pramipexole Extended Release Dosage Regimens in Parkinson's Disease
Abstract
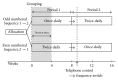
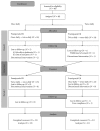
This open-label study aimed to compare once-daily and twice-daily pramipexole extended release (PER) treatment in Parkinson's disease (PD). PD patients on dopamine agonist therapy, but with unsatisfactory control, were enrolled. Existing agonist doses were switched into equivalent PER doses. Subjects were consecutively enrolled into either once-daily-first or twice-daily-first groups and received the prescribed amount in one or two, respectively, daily doses for 8 weeks. For the second period, subjects switched regimens in a crossover manner. The forty-four patients completed a questionnaire requesting preference during their last visit. We measured the UPDRS-III, Hoehn and Yahr stages (H&Y) in medication-on state, Parkinson's disease sleep scale (PDSS), and Epworth Sleepiness Scale. Eighteen patients preferred a twice-daily regimen, 12 preferred a once-daily regimen, and 14 had no preference. After the trial, 14 subjects wanted to be on a once-daily regimen, 25 chose a twice-daily regimen, and 5 wanted to maintain the prestudy regimen. Main reasons for choosing the twice-daily regimen were decreased off-duration, more tolerable off-symptoms, and psychological stability. The mean UPDRS-III, H&Y, and PDSS were not different. Daytime sleepiness was significantly high in the once-daily regimen, whereas nocturnal hallucinations were more common in the twice-daily. Multiple dosing should be considered if once-daily dosing is unsatisfactory. This study is registered as NCT01515774 at ClinicalTrials.gov.
Conflict of interest statement
Beomseok Jeon has received funding for travel from Korea Research-Based Pharmaceutical Industry Association and Korean Pharmaceutical Manufacturers Association and has received research support as PI from Ipsen, Norvartis, Boehringer Ingelheim, the Korea Health 21 R&D project, Ministry of Health & Welfare, Republic of Korea, the National Research Foundation of Korea (NRF), Ministry of Education, Science and Technology, ABRC (Advanced Biometric Research Center), KOSEF (Korean Science and Engineering Foundation), Seoul National University Hospital, the Mr. Chung Suk-Gyoo and Sinyang Cultural Foundation, and the Song Foundation. Ji Young Yun, Young Eun Kim, Han-Joon Kim, and Hui-Jun Yang report no disclosures.
Figures

Similar articles
-
Comparison of once-daily versus twice-daily combination of ropinirole prolonged release in Parkinson's disease.BMC Neurol. 2013 Sep 2;13:113. doi: 10.1186/1471-2377-13-113. BMC Neurol. 2013. PMID: 24004540 Free PMC article. Clinical Trial.
-
Efficacy and safety of a once-daily extended-release formulation of pramipexole switched from an immediate-release formulation in patients with advanced Parkinson's disease: results from an open-label study.Drug Res (Stuttg). 2013 Dec;63(12):639-43. doi: 10.1055/s-0033-1351257. Epub 2013 Jul 24. Drug Res (Stuttg). 2013. PMID: 23884661 Clinical Trial.
-
The range and nature of sleep dysfunction in untreated Parkinson's disease (PD). A comparative controlled clinical study using the Parkinson's disease sleep scale and selective polysomnography.J Neurol Sci. 2006 Oct 25;248(1-2):158-62. doi: 10.1016/j.jns.2006.05.004. Epub 2006 Jun 15. J Neurol Sci. 2006. PMID: 16780888
-
Pramipexole extended release: in Parkinson's disease.CNS Drugs. 2010 Apr;24(4):327-36. doi: 10.2165/11204570-000000000-00000. CNS Drugs. 2010. PMID: 20297857 Review.
-
Pramipexole extended-release: a review of its use in patients with Parkinson's disease.Drugs. 2014 Dec;74(18):2175-90. doi: 10.1007/s40265-014-0322-5. Drugs. 2014. PMID: 25385556 Review.
Cited by
-
Therapeutic strategies in the early stages of Parkinson's disease: a cross-sectional evaluation of 15 years' experience with a large cohort of Romanian patients.Neuropsychiatr Dis Treat. 2019 Apr 5;15:831-838. doi: 10.2147/NDT.S197630. eCollection 2019. Neuropsychiatr Dis Treat. 2019. PMID: 31040682 Free PMC article.
References
-
- Pahwa R., Factor S. A., Lyons K. E., et al. Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66(7):983–995. doi: 10.1212/01.wnl.0000215250.82576.87. - DOI - PubMed
-
- Obeso J. A., Rodriguez-Oroz M. C., Chana P., et al. The evolution and origin of motor complications in Parkinson's disease. Neurology. 2000;55(11, supplement 4):S13–S23. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

