Twice-Daily versus Once-Daily Pramipexole Extended Release Dosage Regimens in Parkinson's Disease
- PMID: 28265478
- PMCID: PMC5318624
- DOI: 10.1155/2017/8518929
Twice-Daily versus Once-Daily Pramipexole Extended Release Dosage Regimens in Parkinson's Disease
Abstract
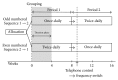
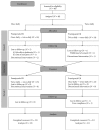
This open-label study aimed to compare once-daily and twice-daily pramipexole extended release (PER) treatment in Parkinson's disease (PD). PD patients on dopamine agonist therapy, but with unsatisfactory control, were enrolled. Existing agonist doses were switched into equivalent PER doses. Subjects were consecutively enrolled into either once-daily-first or twice-daily-first groups and received the prescribed amount in one or two, respectively, daily doses for 8 weeks. For the second period, subjects switched regimens in a crossover manner. The forty-four patients completed a questionnaire requesting preference during their last visit. We measured the UPDRS-III, Hoehn and Yahr stages (H&Y) in medication-on state, Parkinson's disease sleep scale (PDSS), and Epworth Sleepiness Scale. Eighteen patients preferred a twice-daily regimen, 12 preferred a once-daily regimen, and 14 had no preference. After the trial, 14 subjects wanted to be on a once-daily regimen, 25 chose a twice-daily regimen, and 5 wanted to maintain the prestudy regimen. Main reasons for choosing the twice-daily regimen were decreased off-duration, more tolerable off-symptoms, and psychological stability. The mean UPDRS-III, H&Y, and PDSS were not different. Daytime sleepiness was significantly high in the once-daily regimen, whereas nocturnal hallucinations were more common in the twice-daily. Multiple dosing should be considered if once-daily dosing is unsatisfactory. This study is registered as NCT01515774 at ClinicalTrials.gov.
Conflict of interest statement
Beomseok Jeon has received funding for travel from Korea Research-Based Pharmaceutical Industry Association and Korean Pharmaceutical Manufacturers Association and has received research support as PI from Ipsen, Norvartis, Boehringer Ingelheim, the Korea Health 21 R&D project, Ministry of Health & Welfare, Republic of Korea, the National Research Foundation of Korea (NRF), Ministry of Education, Science and Technology, ABRC (Advanced Biometric Research Center), KOSEF (Korean Science and Engineering Foundation), Seoul National University Hospital, the Mr. Chung Suk-Gyoo and Sinyang Cultural Foundation, and the Song Foundation. Ji Young Yun, Young Eun Kim, Han-Joon Kim, and Hui-Jun Yang report no disclosures.
Figures

References
-
- Pahwa R., Factor S. A., Lyons K. E., et al. Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66(7):983–995. doi: 10.1212/01.wnl.0000215250.82576.87. - DOI - PubMed
-
- Obeso J. A., Rodriguez-Oroz M. C., Chana P., et al. The evolution and origin of motor complications in Parkinson's disease. Neurology. 2000;55(11, supplement 4):S13–S23. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

