Germline-specific labeling of the somatic chromosomes by protein phosphatase 2A and histone H3S28 phosphorylation in Acricotopus lucidus
- PMID: 28265764
- PMCID: PMC5610207
- DOI: 10.1007/s00709-017-1092-1
Germline-specific labeling of the somatic chromosomes by protein phosphatase 2A and histone H3S28 phosphorylation in Acricotopus lucidus
Abstract
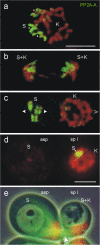
Additional chromosomes limited to the germline (=Ks) were established as a special form of germline-soma differentiation in the Orthocladiinae, a subfamily of the Chironomidae (Bauer and Beermann in Z Naturforsch 7b: 557-563, 1952). The Ks together with the somatic chromosomes (=Ss) pass through a complex chromosome cycle with elimination at mitosis and a monopolar migration of all Ks. The dissimilar behavior of Ks and Ss in these exceptional mitoses initiated the search for differential chromosome marks in the orthocladiid Acricotopus lucidus. The search, using immunofluorescence, revealed that in metaphases of male gonial mitoses, and both meiotic divisions, the Ss are fully labeled by protein phosphatase 2A (PP2A) and histone H3S28ph, while in metaphases of somatic cells both marks were detected only at the centromeres of the Ss. In another orthocladiid, Psectrocladius obvius, the same labeling pattern of the Ss as in A. lucidus was established for H3S28ph, but not for PP2A, which was localised solely at the centromeres. In Chironomus nuditaris, a species possessing no Ks, PP2A and H3S28ph signals were always restricted to the centromeres. High levels of H3K4me3, a marker of transcriptionally competent chromatin, were detected on the Ss in metaphases I of C. nuditaris, while in both orthocladiids, the Ss in metaphases I were devoid of H3K4me3 signals. This strongly supports an earlier idea of a silencing of the Ss in male meiosis of A. lucidus suggesting the possibility of extending this concept to the Orthocladiinae. The germline-soma differentiation in A. lucidus is not only made apparent by the occurrence of Ks but also by a germline-specific labeling of the Ss by PP2A and H3S28ph.
Keywords: Germline-specific chromosome marks; Germline–soma differentiation; Orthocladiinae; Somatic chromosomes.
Conflict of interest statement
Ethical approval
All national and institutional guidelines for the care and use of animals were followed.
Conflict of interest
The author declares that he has no conflict of interest.
Figures





Similar articles
-
Evolutionary Perspectives on Germline-Restricted Chromosomes in Flies (Diptera).Genome Biol Evol. 2021 Jun 8;13(6):evab072. doi: 10.1093/gbe/evab072. Genome Biol Evol. 2021. PMID: 33890671 Free PMC article. Review.
-
Germ line-limited and somatic chromosomes of Acricotopus lucidus differ in distribution and timing of alterations of histone modifications in male gonial mitosis and meiosis.Chromosome Res. 2012 Aug;20(6):717-34. doi: 10.1007/s10577-012-9308-x. Epub 2012 Aug 22. Chromosome Res. 2012. PMID: 22911004
-
Loss of centromeric histone H2AT120 phosphorylation accompanies somatic chromosomes inactivation in the aberrant spermatocytes of Acricotopus lucidus (Diptera, Chironomidae).Protoplasma. 2016 Jan;253(1):211-6. doi: 10.1007/s00709-015-0801-x. Epub 2015 Mar 28. Protoplasma. 2016. PMID: 25820679
-
GTPase Ran strongly accumulates at the kinetochores of somatic chromosomes in the spermatogonial mitoses of Acricotopus lucidus (Diptera, Chironomidae).Protoplasma. 2014 Jul;251(4):979-84. doi: 10.1007/s00709-013-0578-8. Epub 2013 Nov 16. Protoplasma. 2014. PMID: 24240516
-
Germline histone dynamics and epigenetics.Curr Opin Cell Biol. 2007 Jun;19(3):257-65. doi: 10.1016/j.ceb.2007.04.015. Epub 2007 Apr 27. Curr Opin Cell Biol. 2007. PMID: 17467256 Review.
Cited by
-
Evolutionary Perspectives on Germline-Restricted Chromosomes in Flies (Diptera).Genome Biol Evol. 2021 Jun 8;13(6):evab072. doi: 10.1093/gbe/evab072. Genome Biol Evol. 2021. PMID: 33890671 Free PMC article. Review.
References
-
- Bauer H, Beermann W. Der Chromosomencyclus der Orthocladiinen (Nematocera, Diptera) Z Naturforsch. 1952;7b:557–563.
-
- Gerbi SA (1986) Unusual movements in sciarid flies. In: Hennig W (ed) Germ-line-soma differentiation. Results and problems of cell differentiation. Springer, New York, pp 71–104 - PubMed
-
- Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M, Okawa K, Iwamatsu A, Okigaki T, Takahashi T, Inagaki M. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem. 1999;274:25543–25549. doi: 10.1074/jbc.274.36.25543. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

