ELF-MF exposure affects the robustness of epigenetic programming during granulopoiesis
- PMID: 28266526
- PMCID: PMC5339735
- DOI: 10.1038/srep43345
ELF-MF exposure affects the robustness of epigenetic programming during granulopoiesis
Abstract
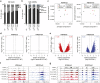
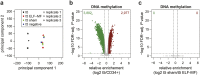
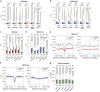
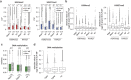
Extremely-low-frequency magnetic fields (ELF-MF) have been classified as "possibly carcinogenic" to humans on the grounds of an epidemiological association of ELF-MF exposure with an increased risk of childhood leukaemia. Yet, underlying mechanisms have remained obscure. Genome instability seems an unlikely reason as the energy transmitted by ELF-MF is too low to damage DNA and induce cancer-promoting mutations. ELF-MF, however, may perturb the epigenetic code of genomes, which is well-known to be sensitive to environmental conditions and generally deranged in cancers, including leukaemia. We examined the potential of ELF-MF to influence key epigenetic modifications in leukaemic Jurkat cells and in human CD34+ haematopoietic stem cells undergoing in vitro differentiation into the neutrophilic lineage. During granulopoiesis, sensitive genome-wide profiling of multiple replicate experiments did not reveal any statistically significant, ELF-MF-dependent alterations in the patterns of active (H3K4me2) and repressive (H3K27me3) histone marks nor in DNA methylation. However, ELF-MF exposure showed consistent effects on the reproducibility of these histone and DNA modification profiles (replicate variability), which appear to be of a stochastic nature but show preferences for the genomic context. The data indicate that ELF-MF exposure stabilizes active chromatin, particularly during the transition from a repressive to an active state during cell differentiation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- SCENIHR. Potential health effects of exposure to electromagnetic fields (EMF). Eur. Comm. [Rep.] EUR (2015).
-
- WHO. Extremely low frequency fields. Vol. 238 (2007).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

