Ethylene-induced transcriptional and hormonal responses at the onset of sugarcane ripening
- PMID: 28266527
- PMCID: PMC5339719
- DOI: 10.1038/srep43364
Ethylene-induced transcriptional and hormonal responses at the onset of sugarcane ripening
Abstract
The effects of ethephon as a sugarcane ripener are attributed to ethylene. However, the role of this phytohormone at the molecular level is unknown. We performed a transcriptome analysis combined with the evaluation of sucrose metabolism and hormone profiling of sugarcane plants sprayed with ethephon or aminoethoxyvinylglycine (AVG), an ethylene inhibitor, at the onset of ripening. The differential response between ethephon and AVG on sucrose level and sucrose synthase activity in internodes indicates ethylene as a potential regulator of sink strength. The correlation between hormone levels and transcriptional changes suggests ethylene as a trigger of multiple hormone signal cascades, with approximately 18% of differentially expressed genes involved in hormone biosynthesis, metabolism, signalling, and response. A defence response elicited in leaves favoured salicylic acid over the ethylene/jasmonic acid pathway, while the upper internode was prone to respond to ethylene with strong stimuli on ethylene biosynthesis and signalling genes. Besides, ethylene acted synergistically with abscisic acid, another ripening factor, and antagonistically with gibberellin and auxin. We identified potential ethylene target genes and characterized the hormonal status during ripening, providing insights into the action of ethylene at the site of sucrose accumulation. A molecular model of ethylene interplay with other hormones is proposed.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

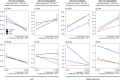
 or 0.01*). n.s. means not statistically significant.
or 0.01*). n.s. means not statistically significant.

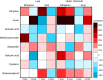





References
-
- Bull T. A. & Glasziou K. T. The evolutionary significance of sugar accumulation in Saccharum. Aust. J. Biol. Sci. 16, 737–742 (1963).
-
- FAOSTAT. FAOSTAT: agricultural data faostat.fao.org (2016).
-
- Segato S. V., Mattiuz C. F. M. & Mozambani A. E. Aspectos fenológicos da cana-de-açúcar in Atualização em produção de cana-de-açúcar (eds Segato S. V., Pinto A. S., Jendiroba E. & Nóbrega J. C. M.) 19–36 (Piracicaba CP 2, 2006).
-
- Cardozo N. P. & Sentelhas P. C. Climatic effects on sugarcane ripening under the influence of cultivars and crop age. Sci. Agric. 70, 449–456 (2013).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

