Experimental Evaluation of Kidney Regeneration by Organ Scaffold Recellularization
- PMID: 28266553
- PMCID: PMC5339865
- DOI: 10.1038/srep43502
Experimental Evaluation of Kidney Regeneration by Organ Scaffold Recellularization
Abstract
The rising number of patients needing renal replacement therapy, alongside the significant clinical and economic limitations of current therapies, creates an imperative need for new strategies to treat kidney diseases. Kidney bioengineering through the production of acellular scaffolds and recellularization with stem cells is one potential strategy. While protocols for obtaining organ scaffolds have been developed successfully, scaffold recellularization is more challenging. We evaluated the potential of in vivo and in vitro kidney scaffold recellularization procedures. Our results show that acellular scaffolds implanted in rats cannot be repopulated with host cells, and in vitro recellularization is necessary. However, we obtained very limited and inconsistent cell seeding when using different infusion protocols, regardless of injection site. We also obtained experimental and theoretical data indicating that uniform cell delivery into the kidney scaffolds cannot be obtained using these infusion protocols, due to the permeability of the extracellular matrix of the scaffold. Our results highlight the major physical barriers that limit in vitro recellularization of acellular kidney scaffolds and the obstacles that must be investigated to effectively advance this strategy for regenerative medicine.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
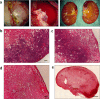
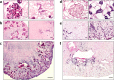
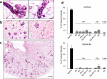

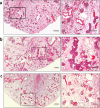
References
-
- Liyanage T. et al.. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 385, 1975–1982 (2015). - PubMed
-
- Couser W. G., Remuzzi G., Mendis S. & Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int 80, 1258–1270 (2011). - PubMed
-
- Winkelmayer W. C., Weinstein M. C., Mittleman M. A., Glynn R. J. & Pliskin J. S. Health economic evaluations: the special case of end-stage renal disease treatment. Med Decis Making 22, 417–430 (2002). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

