Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity
- PMID: 28266779
- PMCID: PMC5573908
- DOI: 10.1111/dom.12932
Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity
Abstract
Aim: The aim of this trial was to investigate the mechanism of action for body weight loss with semaglutide.
Materials and methods: This randomised, double-blind, placebo-controlled, two-period crossover trial investigated the effects of 12 weeks of treatment with once-weekly subcutaneous semaglutide, dose-escalated to 1.0 mg, in 30 subjects with obesity. Ad libitum energy intake, ratings of appetite, thirst, nausea and well-being, control of eating, food preference, resting metabolic rate, body weight and body composition were assessed.
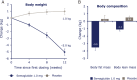
Results: After a standardised breakfast, semaglutide, compared with placebo, led to a lower ad libitum energy intake during lunch (-1255 kJ; P < .0001) and during the subsequent evening meal ( P = .0401) and snacks ( P = .0034), resulting in a 24% reduction in total energy intake across all ad libitum meals throughout the day (-3036 kJ; P < .0001). Fasting overall appetite suppression scores were improved with semaglutide vs placebo, while nausea ratings were similar. Semaglutide was associated with less hunger and food cravings, better control of eating and a lower preference for high-fat foods. Resting metabolic rate, adjusted for lean body mass, did not differ between treatments. Semaglutide led to a reduction from baseline in mean body weight of 5.0 kg, predominantly from body fat mass.
Conclusion: After 12 weeks of treatment, ad libitum energy intake was substantially lower with semaglutide vs placebo with a corresponding loss of body weight observed with semaglutide. In addition to reduced energy intake, likely mechanisms for semaglutide-induced weight loss included less appetite and food cravings, better control of eating and lower relative preference for fatty, energy-dense foods.
Keywords: Body composition; Energy regulation; GLP-1 analogue; Glucagon-like peptide-1; Randomised trial; Semaglutide; Type 2 diabetes; Visual analogue scale.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

