Recent advances in genetic modification of adenovirus vectors for cancer treatment
- PMID: 28266780
- PMCID: PMC5448613
- DOI: 10.1111/cas.13228
Recent advances in genetic modification of adenovirus vectors for cancer treatment
Abstract
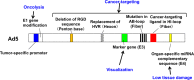
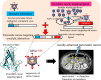
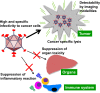
Adenoviruses are widely used to deliver genes to a variety of cell types and have been used in a number of clinical trials for gene therapy and oncolytic virotherapy. However, several concerns must be addressed for the clinical use of adenovirus vectors. Selective delivery of a therapeutic gene by adenovirus vectors to target cancer is precluded by the widespread distribution of the primary cellular receptors. The systemic administration of adenoviruses results in hepatic tropism independent of the primary receptors. Adenoviruses induce strong innate and acquired immunity in vivo. Furthermore, several modifications to these vectors are necessary to enhance their oncolytic activity and ensure patient safety. As such, the adenovirus genome has been engineered to overcome these problems. The first part of the present review outlines recent progress in the genetic modification of adenovirus vectors for cancer treatment. In addition, several groups have recently developed cancer-targeting adenovirus vectors by using libraries that display random peptides on a fiber knob. Pancreatic cancer-targeting sequences have been isolated, and these oncolytic vectors have been shown by our group to be associated with a higher gene transduction efficiency and more potent oncolytic activity in cell lines, murine models, and surgical specimens of pancreatic cancer. In the second part of this review, we explain that combining cancer-targeting strategies can be a promising approach to increase the clinical usefulness of oncolytic adenovirus vectors.
Keywords: Adenovirus; fiber knob; library; pancreatic cancer; targeting.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
References
-
- Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 – an update. J Gene Med 2013; 15: 65–77. - PubMed
-
- Bergelson JM, Cunningham JA, Droguett G et al Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 1997; 275: 1320–3. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

