Personalized treatment in heart transplantation
- PMID: 28266942
- PMCID: PMC5646374
- DOI: 10.1097/MOT.0000000000000406
Personalized treatment in heart transplantation
Abstract
Purpose of review: We are entering the era of personalized medicine, in which pharmacogenomics and biomarker-based assays can be used to tailor diagnostic tests and drug therapies to individual patients. This new approach to patient-specific care offers the potential to maximize the efficacy of available medical treatments while reducing the incidence of adverse side effects. Here, we present approaches to personalize the care of heart transplant recipients.
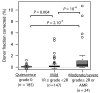
Recent findings: Four strategies for personalized posttransplant care are described, including use of pharmacogenomic data to individualize the use of immunosuppressive drugs, immune monitoring to prevent acute rejection while reducing the long-term consequences of over immunosuppression, noninvasive surveillance for acute rejection, and targeted prophylaxis against opportunistic infections.
Summary: The long-term survival of heart transplant recipients is limited by side effects of immunosuppressive drugs, including infectious complications, renal dysfunction, and malignancy. We discuss strategies to maximize the benefits of immunosuppressive and prophylactic therapies while minimizing their long-term toxicities.
Conflict of interest statement
Figures

References
-
- Lund LH, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant. 2016;35(10):1158–69. - PubMed
-
- Aspinall MG, Hamermesh RG. Realizing the promise of personalized medicine. Harv Bus Rev. 2007;85(10):108–17. 65. - PubMed
-
- Piquette-Miller M, Grant DM. The art and science of personalized medicine. Clin Pharmacol Ther. 2007;81(3):311–5. - PubMed
-
- Evans WE, McLeod HL. Pharmacogenomics--drug disposition, drug targets, and side effects. N Engl J Med. 2003;348(6):538–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

