Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons
- PMID: 28267064
- PMCID: PMC5435541
- DOI: 10.1097/j.pain.0000000000000894
Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons
Abstract
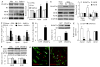
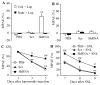
Opioids are the gold standard for pharmacological treatment of neuropathic pain, but their analgesic effects are unsatisfactory in part due to nerve injury-induced downregulation of opioid receptors in dorsal root ganglia (DRG) neurons. How nerve injury drives such downregulation remains elusive. DNA methyltransferase (DNMT)-triggered DNA methylation represses gene expression. We show here that blocking the nerve injury-induced increase in DRG DNMT3a (a de novo DNMT) rescued the expression of Oprm1 and Oprk1 mRNAs and their respective encoding mu-opioid receptor (MOR) and kappa-opioid receptor (KOR) proteins in the injured DRG. Blocking this increase also prevented the nerve injury-induced increase in DNA methylation in the promoter and 5'-untranslated region of the Oprm1 gene in the injured DRG, restored morphine or loperamide (a peripheral acting MOR preferring agonist) analgesic effects, and attenuated the development of their analgesic tolerance under neuropathic pain conditions. Mimicking this increase reduced the expression of Oprm1 and Oprk1 mRNAs and their coding MOR and KOR in DRG and augmented MOR-gated neurotransmitter release from the primary afferents. Mechanistically, DNMT3a regulation of Oprm1 gene expression required the methyl-CpG-binding protein 1, MBD1, as MBD1 knockout resulted in the decreased binding of DNMT3a to the Oprm1 gene promoter and blocked the DNMT3a-triggered repression of Oprm1 gene expression in DRG neurons. These data suggest that DNMT3a is required for nerve injury-induced and MBD1-mediated epigenetic silencing of the MOR and KOR in the injured DRG. DNMT3a inhibition may serve as a promising adjuvant therapy for opioid use in neuropathic pain management.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures
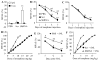
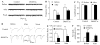

References
-
- Anesti AM, Coffin RS. Delivery of RNA interference triggers to sensory neurons in vivo using herpes simplex virus. Expert Opin Biol Ther. 2010;10:89–103. - PubMed
-
- Ataka T, Kumamoto E, Shimoji K, Yoshimura M. Baclofen inhibits more effectively C-afferent than Adelta-afferent glutamatergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Pain. 2000;86:273–282. - PubMed
-
- Bekhit MH. Opioid-induced hyperalgesia and tolerance. Am J Ther. 2010;17:498–510. - PubMed
-
- Chen T, Ueda Y, Xie S, Li E. A novel Dnmt3a isoform produced from an alternative promoter localizes to euchromatin and its expression correlates with active de novo methylation. J Biol Chem. 2002;277:38746–38754. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

