External beam radiotherapy for unresectable hepatocellular carcinoma
- PMID: 28267205
- PMCID: PMC6464365
- DOI: 10.1002/14651858.CD011314.pub2
External beam radiotherapy for unresectable hepatocellular carcinoma
Abstract
Background: Hepatocellular carcinoma is the most common liver neoplasm, the sixth most common cancer worldwide, and the third most common cause of cancer mortality. Moreover, its incidence has increased dramatically in the past decade. While surgical resection and liver transplantation are the main curative treatments, only around 20% of people with early hepatocellular carcinoma may benefit from these therapies. Current treatment options for unresectable hepatocellular carcinoma include various ablative and transarterial therapies in addition to the drug sorafenib.
Objectives: To assess the benefits and harms of external beam radiotherapy in the management of localised unresectable hepatocellular carcinoma.
Search methods: We searched the Cochrane Hepato-Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, MEDLINE (OvidSP), Embase (OvidSP), Science Citation Index Expanded (Web of Science), and clinicaltrials.gov registry. We also checked reference lists of primary original studies and review articles manually for further related articles (cross-references) up to October 6, 2016.
Selection criteria: Eligible studies included all randomised clinical trials comparing external beam radiotherapy either as a monotherapy or in combination with other systemic or locoregional therapies versus placebo, no treatment, or other systemic or locoregional therapies for people with unresectable hepatocellular carcinoma.
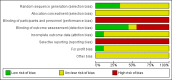
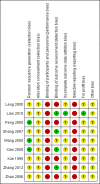
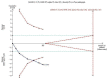
Data collection and analysis: We used standard methodological procedures expected by Cochrane. We used a random-effects model as well as a fixed-effect model meta-analysis but in case of discrepancy between the two models (e.g. one giving a significant intervention effect, the other no significant intervention effect), we reported both results; otherwise, we reported only the results from the fixed-effect model meta-analysis. We assessed risk of bias of the included trials using predefined risk of bias domains; assessed risks of random errors with Trial Sequential Analysis; and presented the review results incorporating the methodological quality of the trials using GRADE.
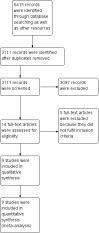
Main results: Nine randomised clinical trials with 879 participants fulfilled our inclusion criteria. All trials were at high risk of bias, and we rated the evidence as low to very low quality. All of the included trials compared combined external beam radiotherapy plus chemoembolisation versus chemoembolisation alone in people with unresectable hepatocellular carcinoma; moreover, three of the trials compared external beam radiotherapy alone versus chemoembolisation alone. All trials were conducted in China. The median age in most of the included trials was around 52 years, and most trial participants were male. The median follow-up duration ranged from one to three years. None of the trials reported data on cancer-related mortality, quality of life, serious adverse events, or time to progression of the tumour. For the comparison of radiotherapy plus chemoembolisation versus chemoembolisation alone, the risk ratio for one-year all-cause mortality was 0.51 (95% confidence interval (CI) 0.41 to 0.62; P < 0.001; 9 trials; low-quality evidence); for complete response rate was 2.14 (95% CI 1.47 to 3.13; P < 0.001; 7 trials; low-quality evidence); and for overall response rate defined as complete response plus partial response was 1.58 (95% CI 1.40 to 1.78; P < 0.001; 7 trials; low-quality evidence), all in favour of combined treatment with external beam radiotherapy plus transarterial chemoembolisation and seemingly supported by our Trial Sequential Analysis. Additionally, the combined treatment was associated with a higher risk of elevated total bilirubin and elevated alanine aminotransferase. The risk ratio for the risk of elevated alanine aminotransferase was 1.41 (95% CI 1.08 to 1.84; P = 0.01; very low-quality evidence), while for elevated total bilirubin it was 2.69 (95% CI 1.34 to 5.40; P = 0.005; very low-quality evidence). For the comparison of radiotherapy versus chemoembolisation, the risk ratio for one-year all-cause mortality was 1.21 (95% CI 0.97 to 1.50; 3 trials; I2 = 0%; very low-quality evidence) which was not supported by our Trial Sequential Analysis.In addition, we found seven ongoing randomised clinical trials evaluating different external beam radiotherapy techniques for people with unresectable hepatocellular carcinoma.
Authors' conclusions: We found very low- and low-quality evidence suggesting that combined external beam radiotherapy and chemoembolisation may be associated with lower mortality and increased complete and overall response rates, despite an increased toxicity as expressed by a higher rise of bilirubin and alanine aminotransferase. A high risk of systematic errors (bias) as well as imprecision and inconsistency suggest that these findings should be considered cautiously and that high-quality trials are needed to assess further the role of external beam radiotherapy for unresectable hepatocellular carcinoma.
Conflict of interest statement
Omar Abdel‐Rahman: none known. Zeinab Elsayed: none known.
Figures








Update of
- doi: 10.1002/14651858.CD011314
References
References to studies included in this review
Leng 2000 {published data only}
-
- Leng ZQ, Liang ZY, Shi S, Hu ZX. Comparison of treatment results of interventional therapy alone, radiotherapy alone, and combined interventional therapy plus radiotherapy for primary hepatic cancer. Chinese Journal of Radiation Oncology 2000;9:99‐101.
Liao 2010 {published data only}
-
- Liao X, He H, Zhou Z, Hu W, Zhu X. Three‐dimensional conformal radiotherapy combined with interventional therapy in treatment of primary hepatocellular carcinoma. Journal of Practical Oncology 2010;25:681‐3.
Peng 2000 {published data only}
-
- Peng KG, Han FS, Liu H, Song MZ. Clinical study of unresectable liver cancer treated by intraoperative hepatic arterial embolization and post‐operative hyperfractionation radiotherapy. Chinese Journal of Radiation Oncology 2000;9:11‐3.
Shang 2007 {published data only}
-
- Shang Y, You XX, Xu HY, Chen MC. Prospective randomized clinical study of transcatheter arterial chemoembolization, combined with three‐dimensional conformal radiotherapy for primary liver cancer: an analysis of 40 cases. Shijie Xiaohua Zazhi 2007;15:3140‐2.
Wang 2000 {published data only}
-
- Wang G, Shen W, Song M, Xu H. Results of combined treatment with transcatheter hepatic arterial chemoembolization and whole‐liver irradiation with the moving strip technique in unresectable hepatocellular carcinoma. International Journal of Clinical Oncology 2000;5:380‐5.
Xiao 2008 {published data only}
-
- Xiao Z, Ouyang T, Yu R, Jiang X, Reng H, Wu Z. Transcatheter arterial chemoembolization combined with 3‐dimensional conformal radiotherapy for patients with unresectable primary hepatic carcinoma. Chinese Journal of Clinical Oncology 2008;35(1):18‐21.
Xue 1995 {published data only}
-
- Xue HZ, Meng GD, Wang YW, Jiang QF. Transcatheter arterial chemoembolization plus radiotherapy in the treatment of hepatocellular carcinoma. Chinese Journal of Radiation Oncology 1995;4:84‐5.
Zhang 2012 {published data only}
-
- Zhang Z, Yang X, Wena M, Wan J. Evaluation of TACE combined with gamma‐knife radiotherapy for primary hepatocellular carcinoma. Journal of Interventional Radiology (China) 2012;7(21):596‐9.
Zhao 2006 {published data only}
-
- Zhao MH, Lang FP, Jiang QA, Ma JJ, Song YX. Three‐dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for inoperable primary liver cancer. Chinese Journal of Radiation Oncology 2006;15:39‐41.
References to studies excluded from this review
Bush 2016 {published data only}
-
- Bush DA, Smith JC, Slater JD, Volk ML, Reeves ME, Cheng J, et al. Randomized clinical trial comparing proton beam radiation therapy with transarterial chemoembolization for hepatocellular carcinoma: results of an interim analysis. International Journal of Radiation Oncology, Biology, Physics 2016;95(1):477‐82. - PubMed
Kang 2014 {published data only}
Lan 2005 {published data only}
-
- Lan DQ, Gong XH, Wei XL. The efficacy analysis of transcatheter hepatic arterial chemoembolization combined with radiotherapy for primary liver cancer. Chinese Journal of Radiation Oncology 2005;14:152‐3.
Li 2003 {published data only}
-
- Li B, Yu J, Wang L, Li C, Zhou T, Zhai L, et al. Study of local three‐dimensional conformal radiotherapy combined with transcatheter arterial chemoembolization for patients with stage III hepatocellular carcinoma. American Journal of Clinical Oncology 2003;26(4):e92‐9. - PubMed
Wang 2006 {published data only}
-
- Wang XH, Li JX, Gao K. Radiotherapy combined with hepatic chemoembolization in the treatment of 54 primary liver cancer cases. Shaxi Yixue Zazhi 2006;35(4):461‐2.
References to ongoing studies
NCT01730937 {published data only}
-
- NCT01730937. Sorafenib tosylate with or without stereotactic body radiation therapy in treating patients with liver cancer [Randomized phase III study of sorafenib versus stereotactic body radiation therapy followed by sorafenib in hepatocellular carcinoma]. clinicaltrials.gov/ct2/show/NCT01730937 (first received November 15, 2012 ).
NCT01901692 {published data only}
-
- NCT01901692. Transarterial chemoembolization plus radiotherapy or sorafenib in hepatocellular carcinoma with major vascular invasion (START) [Randomized phase II trial comparing transarterial chemoembolization plus external beam radiotherapy versus sorafenib in patients with hepatocellular carcinoma with major vascular invasion]. clinicaltrials.gov/ct2/show/NCT01901692 (first received July 14, 2013 ).
NCT01963429 {published data only}
-
- NCT01963429. Comparison between radiofrequency ablation and hypofractionated proton beam radiation for recurrent/residual HCC [A randomized phase III study of the comparison between radiofrequency ablation and hypofractionated proton beam radiation in patients with recurrent/residual small hepatocellular carcinoma (APROH Study)]. clinicaltrials.gov/ct2/show/NCT01963429 (first received October 13, 2013 ).
NCT02323360 {published data only}
-
- NCT02323360. A trial on SBRT after incomplete TAE or TACE versus exclusive TAE or TACE for treatment of inoperable HCC [A randomised phase III trial on stereotactic body radiotherapy (SBRT) after incomplete transcatheter arterial embolization (TAE) or chemoembolization (TACE) versus exclusive TAE or TACE for inoperable hepatocellular carcinoma (HCC)]. clinicaltrials.gov/ct2/show/NCT02323360 (first received December 11, 2014 ).
NCT02640924 {published data only}
-
- NCT02640924. Proton radiotherapy versus radiofrequency ablation for patients with medium or large hepatocellular carcinoma [Proton beam radiotherapy versus switching control radiofrequency ablation for patients with medium (>3, ≦5 cm) or large (>5, ≦7cm) treatment‐naive hepatocellular carcinoma]. clinicaltrials.gov/ct2/show/NCT02640924 (first received December 7, 2015 ).
NCT02762266 {published data only}
-
- NCT02762266. Transarterial chemoembolization compared with stereotactic body radiation therapy or stereotactic ablative radiation therapy in treating patients with residual or recurrent liver cancer undergone initial transarterial chemoembolization [International randomized study of transarterial chemoembolization (TACE) versus stereotactic body radiotherapy (SBRT) / stereotactic ablative radiotherapy (SABR) for residual or recurrent hepatocellular carcinoma after initial TACE]. clinicaltrials.gov/ct2/show/NCT02762266 (first received April 5, 2016 ).
NCT02794337 {published data only}
-
- NCT02794337. TACE vs TACE+SBRT for unresectable hepatocellular cancer (TACE‐SBRT) [Integrated phase II/III randomized control trial of transarterial chemoembolisation alone or in combination with stereotactic body radiation in patients with unresectable hepatocellular cancer]. clinicaltrials.gov/ct2/show/NCT02794337 (first received March 8, 2016 ).
Additional references
Abdel‐Rahman 2013
-
- Abdel‐Rahman O, Elsayed Z. Combination trans arterial chemoembolization (TACE) plus sorafenib for the management of unresectable hepatocellular carcinoma: a systematic review of the literature. Digestive Diseases and Sciences 2013; Vol. 58, issue 12:3389‐96. [DOI: 10.1007/s10620-0132872-x] - DOI - PubMed
Abdel‐Rahman 2016
Andolino 2011
-
- Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. International Journal of Radiation Oncology * Biology * Physics 2011;81(4):e447‐53. - PubMed
Balshem 2011
-
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines 3: rating the quality of evidence ‐ introduction. Journal of Clinical Epidemiology 2011;64:401‐6. - PubMed
Ben‐Josef 2005
-
- Ben‐Josef E, Normolle D, Ensminger WD, Walker S, Tatro D, Haken RK, et al. Phase II trial of high‐dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. Journal of Clinical Oncology 2005;23(34):8739‐47. - PubMed
Brok 2008
-
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. - PubMed
Brok 2009
-
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. - PubMed
Bruix 2011
Bujold 2013
-
- Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RKS, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. Journal of Clinical Oncology 2013;31(13):1631‐9. - PubMed
Bush 2011
-
- Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high‐dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer 2011;117(13):3053‐9. - PubMed
Cárdenes 2009
-
- Cárdenes HR. Role of stereotactic body radiotherapy in the management of primary hepatocellular carcinoma. Rationale, technique and results. Clinical Translational Oncology 2009;11(5):276‐83. - PubMed
Dawson 2011
-
- Dawson L. Overview: Where does radiation therapy fit in the spectrum of liver cancer local‐regional therapies?. Seminars in Radiation Oncology 2011;21(4):241‐6. - PubMed
DeMets 1987
-
- DeMets DL. Methods of combining randomised clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
EASL/EORTC 2012
-
- European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer. EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of Hepatology 2012;56(4):908‐43. - PubMed
Egger 1997
Eisenhauer 2009
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45(2):228‐47. - PubMed
Globocan 2012
-
- International Agency for Research on Cancer. Globocan 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. globocan.iarc.fr/Default.aspx (accessed 20 June 2016).
Gluud 2017
-
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2017, Issue 2. Art. No.: LIVER.
GRADEpro 2008 [Computer program]
-
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011a
-
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence ‐‐ study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence ‐‐ publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011f
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Guyatt 2011g
-
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] - PubMed
Guyatt 2013
-
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [PUBMED: 22542023] - PubMed
Guyatt 2013a
-
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables ‐‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [PUBMED: 22609141] - PubMed
Guyatt 2013b
-
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles ‐‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66(2):173‐83. [PUBMED: 23116689] - PubMed
Guyatt 2013c
-
- Guyatt G, Andrews J, Oxman AD, Alderson P, Dahm P, Falck‐Ytter Y, et al. GRADE guidelines: 15. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 2013;66(7):719‐25. - PubMed
Hassan 2011
-
- Hassan M, Kaseb A. Epidemiology and pathogenesis of hepatocellular carcinoma. Hepatocellular Carcinoma: Targeted Therapy and Multidisciplinary Care. Springer, 2011:15‐25.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffe 2010
-
- Hoffe SE, Finkelstein SE, Russell MS, Shridhar R. Nonsurgical options for hepatocellular carcinoma: evolving role of external beam radiotherapy. Cancer Control 2010;17(2):100‐10. - PubMed
Hollis 1999
Hong 2016
-
- Hong TS, Wo JY, Yeap BY, Ben‐Josef E, McDonnell EI, Blaszkowsky LS, et al. Multi‐institutional phase II study of high‐dose hypofractionated proton beam therapy in patients with localized, unresectable hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Journal of Clinical Oncology 2016;34(5):460‐8. - PMC - PubMed
Huo 2015
-
- Huo Y, Eslick GD. Transcatheter arterial chemoembolization plus radiotherapy compared with chemoembolization alone for hepatocellular carcinoma: a systematic review and meta‐analysis. JAMA Oncology 2015;1(6):756‐65. - PubMed
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997.
Jihye 2012
Kawashima 2005
-
- Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. Journal of Clinical Oncology 2005;23(9):1839‐46. - PubMed
Kim 2015
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Klein 2013
-
- Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. International Journal of Radiation Oncology * Biology * Physics 2013;87(1):22‐32. - PubMed
Lee 2014
Lundh 2017
Ma 2010
-
- Ma S, Jiao B, Liu X, Yi H, Kong D, Gao L, et al. Approach to radiation therapy in hepatocellular carcinoma. Cancer Treatment Reviews 2010;36(2):157‐63. - PubMed
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20:641‐54. - PubMed
Maffione 2013
-
- Maffione AM, Ferretti A, Vinjamuri S, Rubello D. The PERCIST criteria: an insightful appraisal. Nuclear Medicine Communications 2013;34(7):619‐20. - PubMed
Maida 2014
Mantel 1959
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;2(4):719‐48. - PubMed
Meng 2009
-
- Meng M‐B, Cui Y‐L, Lu Y, She B, Chen Y, Guan Y‐S, et al. Transcatheter arterial chemoembolization in combination with radiotherapy for unresectable hepatocellular carcinoma: a systematic review and meta‐analysis. Radiotherapy and Oncology 2009;92(2):184‐94. - PubMed
Merle 2009
-
- Merle P, Mornex F, Trepo C. Innovative therapy for hepatocellular carcinoma: three‐dimensional high‐dose photon radiotherapy. Cancer Letters 2009;286(1):129‐33. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Mustafa 2013
-
- Mustafa RA, Santesso N, Brozek J, Akl EA, Walter SD, Norman G, et al. The GRADE approach is reproducible in assessing the quality of evidence of quantitative evidence syntheses. Journal of Clinical Epidemiology 2013;66(7):736‐42; quiz 742.e1‐5. [PUBMED: 23623694] - PubMed
Oliveri 2011
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riaz 2011
Royle 2003
-
- Royle P, Milne R. Literature searching for randomised controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Savović 2012
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised, controlled trials. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Seong 2009
Taefi 2013
Thorlund 2009
-
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. - PubMed
Thorlund 2010
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 20 June 2016).
TSA 2011 [Computer program]
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Tsai 2013
-
- Tsai CL, Koong AC, Hsu FM, Graber M, Chen IS, Cheng JC. Biomarker studies on radiotherapy to hepatocellular carcinoma. Oncology 2013;84(Suppl 1):64‐8. - PubMed
Ursino 2012
-
- Ursino S, Greco C, Cartei F, Colosimo C, Stefanelli A, Cacopardo B, et al. Radiotherapy and hepatocellular carcinoma: update and review of the literature. European Review of Medical and Pharmacological Sciences 2012;16(11):1599‐604. - PubMed
Venook 2010
-
- Venook AP, Papandreou C, Furuse J, Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010;15(Suppl 4):5‐13. - PubMed
Weis 2013
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2009
Wigg 2010
-
- Wigg AJ, Palumbo K, Wigg DR. Radiotherapy for hepatocellular carcinoma: systematic review of radiobiology and modelling projections indicate reconsideration of its use. Journal of Gastroenterology and Hepatology 2010;25(4):664‐71. - PubMed
Wood 2008
Yau 2008
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

