Multicenter phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma
- PMID: 28267244
- PMCID: PMC5448600
- DOI: 10.1111/cas.13230
Multicenter phase II study of nivolumab in Japanese patients with relapsed or refractory classical Hodgkin lymphoma
Abstract
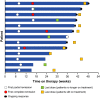
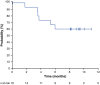
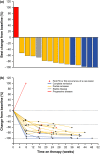
Overexpression of programmed death-1 (PD-1) ligands contributes to an immunosuppressive microenvironment. Nivolumab is a PD-1-blocking antibody that inhibits the PD-1 pathway and showed good efficacy in several types of malignancy. This phase II study examined the efficacy and safety of nivolumab in 17 Japanese patients with refractory/relapsed classical Hodgkin lymphoma previously treated with brentuximab vedotin. Sixteen patients were included in efficacy analyses and 17 in safety analyses. The primary endpoint was the centrally assessed objective response rate (ORR). The study was commenced in March 2015. We report data obtained at a cutoff of 16 March 2016, at which time 11 patients were still receiving nivolumab. The median (range) duration of treatment and follow-up were 7.0 (1.4-10.6) months and 9.8 (6.0-11.1) months, respectively. All 17 patients had previously received brentuximab vedotin. The ORR was 81.3% (95% confidence interval [CI]: 54.4-96.0%; 13/16 patients), with complete remission and partial remission in 4 and 9 patients, respectively. The overall survival (OS) and progression-free survival (PFS) rates at 6 months were 100 and 60.0% (95% CI: 31.8-79.7%), respectively; the median OS and PFS were not reached. The most common adverse events (AE) were pyrexia (41.2%), pruritus (35.3%), rash (35.3%) and hypothyroidism (29.4%). Four patients (23.5%) experienced grade 3 or 4 AE, but most AE were of grade 1 or 2. In conclusion, nivolumab is a potentially effective and tolerable treatment option for Japanese patients with relapsed/refractory classical Hodgkin lymphoma previously treated with brentuximab vedotin.
Keywords: Hodgkin lymphoma; Japanese; immunotherapy; nivolumab; programmed death-1.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
References
-
- Makita S, Maruyama D, Maeshima AM et al Clinical features and outcomes of 139 Japanese patients with Hodgkin lymphoma. Int J Hematol 2016; 104: 233–44. - PubMed
-
- Ogura M, Itoh K, Kinoshita T et al Phase II study of ABVd therapy for newly diagnosed clinical stage II–IV Hodgkin lymphoma: Japan Clinical Oncology Group study (JCOG 9305). Int J Hematol 2010; 92: 713–24. - PubMed
-
- Kewalramani T, Nimer SD, Zelenetz AD et al Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin's disease or aggressive non‐Hodgkin's lymphoma. Bone Marrow Transplant 2003; 32: 673–9. - PubMed
-
- Alley SC, Okeley NM, Senter PD. Antibody‐drug conjugates: Targeted drug delivery for cancer. Curr Opin Chem Biol 2010; 14: 529–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

